If this symbol - “ω” - is not a lower case omega inside quotation marks you should go the Browser Selection Considerations page.
Volume 104, Number 2, March – April 1999
Journal of Research of the National Institute of Standards and Technology
[J. Res. Natl. Inst. Stand. Technol. 104, 147 (1999)]
Crystal Structures and Reference Powder Patterns of BaR2ZnO5 (R=La, Nd, Sm, Eu, Gd, Dy, Ho, Y, Er , and Tm )
J. A. Kaduk
Amoco Corporation,
Naperville, IL 60566
W. Wong-Ng
National Institute of Standards and Technology,
Gaithersburg, MD 20899-0001
W. Greenwood and J. Dillingham
Geology Department, University of Maryland,
College Park, MD 20742
B. H. Toby
National Institute of Standards and Technology,
Gaithersburg, MD 20899-0001
Abstract
Reference x-ray powder patterns and the crystal structures of the lanthanide compounds, BaR2ZnO5, in which R=La, Nd, Sm, Eu, Gd, Dy, Ho, Y, Er, or Tm, were determined by the x-ray Rietveld refinement technique. A structural trend was confirmed for this series of compounds. The compounds with smaller ionic radii (R=Sm, Eu, Gd, Dy, Ho, Y, Er, or Tm) are isostructural to the orthorhombic “green phase” (BaY2CuO5). The lattice parameters for compounds with R=Tm to Sm range from a =7.01855(9) Å to 7.20452(14) Å , b=12.25445 (17) Å to 12.5882(2) Å , and c=5.6786(14) Å to 5.81218(11) Å , respectively. R is seven-fold coordinated inside a monocapped trigonal prism. These prisms share edges to form wave-like chains parallel to the long b-axis. The BaR2ZnO5 compounds which contain larger size R (La and Nd ) crystallize in the tetragonal space group 14/mcm. The lattice parameters are a=6.90982(10) and c=11.5977(2) Å for BaLa2ZnO5, and a=6.75979(5) Å and c=11.54560(12) Å for BaNd2ZnO5. The structure consists of ZnO4 tetrahedra (instead of planar CuO4 groups as found in BaR2CuO5) with 10-fold coordinated bicapped square prismatic Ba and 8-fold coordinated bicapped trigonal prismatic R ions between them. The reference x-ray powder patterns will be submitted to the Powder Diffraction File (PDF).
Keywords: BaR.2ZnO5 (R=lanthanides); crystal structure; x-ray and neutron Rietveld refinements; x-ray reference powder patterns.
Accepted: February 8, 1999
Available in PDF for printing from the NIST Virtual Library. Go!
1. Introduction
Extensive structural and property investigations involving substitution of Cu in the Ba2RCu3O6+x system by various transition metals including Ti, Cr, Mn, Fe, Co, Ni, Au, and Zn have been carried out in order to understand the correlations between superconducting properties and crystal chemistry [1 – 4]. When the Cu2+ (nine 3d electrons) of Ba2YCu3O6+x is completely substituted by Zn2+(ten 3d electrons), the sample does not become superconducting, presumably the result of filling of electronic bands. A strong correlation between superconductivity and electronic and magnetic properties of the substituting elements was reported by Xiao et al. [1]. Therefore, studies of the structure and properties of the Zn-analogs should enhance understanding of the factors contributing to superconductivity.
Successful replacement of Cu by Zn in the lanthanide Ba-R-Cu-O system has been described [5 – 9]. According to Michel et al. [5 – 8], selected barium lanthanum zinc oxides apparently isostructural to the “ green phases” , BaR2CuO5 [2], can be prepared. The structures of the La, Nd, and Y-compounds have been studied using x-ray powder diffraction [5,6] and neutron powder diffraction methods [9]. It was found that, while the structures of the La and Nd analogs are tetragonal, the Y-compound is orthorhombic. Neutron diffraction studies of selected lanthanide analogs (Dy, Ho, Y, and Er) have also been reported [9]. High neutron absorption cross sections meant that compounds containing the lanthanide ions with larger ionic radius such as Sm, Eu, and Gd were not studied. Michel and Raveau [8] further reported that, while in the Cu-containing system BaR2CuO5 the orthorhombic structure can be prepared for R=Er, Tm, Yb and Lu, attempts to replace Cu with Zn did not succeed for these compounds. Recently we reported that the Er-analog of BaR2ZnO5 has been prepared [9].
As the x-ray powder diffraction technique is of primary importance for phase characterization, extensive coverage by accurate reference diffraction patterns of superconductor and related phases in the Powder Diffraction File (PDF) [10] is essential for the high-Tc superconductivity community. Presently, no reference diffraction pattern other than R=La is available in the PDF for phase identification for BaR2ZnO5.
The main goals of this investigation were: to supplement the reference diffraction patterns and crystal structures of the BaR2ZnO5 series by using x-ray Rietveld refinement techniques [11]; to determine the structural details of the analogs with R=Sm, Eu, and Gd; and to investigate the possibility of preparing the analogs of the lanthanide ions with smaller ionic radius (R=Tm, Yb, and Lu).
2. Experimental Details
2. 1. Sample Preparation
Eleven polycrystalline samples of the BaR2ZnO5 series (R=La, Nd, Sm, Eu, Gd, Dy, Ho, Y, Er, Tm, Yb, and Lu) were prepared by a solid-state sintering method. Well-mixed stoichiometric powders of BaCO3, R2O3, and ZnO were compacted by pressing the powder in a pelletizing die at about 0.3 GPa. The compacted powders were heated in air according to the schedule shown in Table 1. Each time the samples were taken out of the furnace, they were reground and repelletized. About 4 g to 5 g of each of the samples was prepared except for the Er, Tm, Yb, and Lu samples, for which only about a 1 g sample was attempted in order to investigate the feasibility of sample preparation. The colors of these materials are also reported in Table 1. When Cu is replaced by Zn, the color of the phases changes from dark green or brown to the much lighter colors of cream, blue, beige, or peach.
Table 1. Heat treatment scheme for BaR2ZnO 5(R= La, Nd, Sm, Eu, Gd, Dy, Ho, Y, Er and Tm). The duration of annealing (number of days) is given in parenthesis.
| R | Color | Temp. (°C) | |||||
| La | white | 850(2) | 950(3) | 1000(1.5) | |||
| Nd | pale blue | 850(2) | 950(3) | 1000(1.5) | |||
| Sm | cream | 850(2) | 950(3) | 1000(1.5) | |||
| Eu | grey | 850(2) | 950(3) | 1000(1.5) | |||
| Gd | beige | 850(2) | 950(3) | 1000(1.5) | |||
| Dy | grey | 850(2) | 950(3) | 1000(1.5) | 1080(5) | 1090(1.5) | |
| Ho | pink | 850(2) | 950(3) | 1000(1.5) | 1080(5) | 1090(1.5) | |
| Y | beige | 850(2) | 950(3) | 1000(1.5) | 1080(5) | 1090(1.5) | 1100(7) |
| Er | lavender | 850(2) | 950(3) | 1000(1.5) | 1080(5) | 1090(1.5) | |
| Tm | cream | 850(2) | 950(3) | 1000(1.5) | 1100(1.5) | 1200(3) | |
| Yb | cream | 850(2) | 950(3) | 1000(1.5) | 1100(1.5) | 1200(3) | |
| Lu | cream | 850(2) | 950(3) | 1000(1.5) | 1100(1.5) | 1200(3) |
2. 2. X-Ray Powder Studies
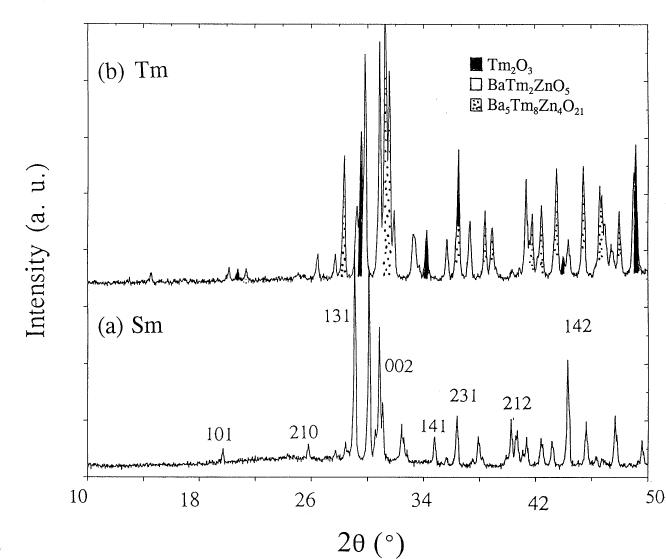
X-ray powder diffraction was used to identify the phases synthesized and to confirm phase purity. The PDF reference x-ray diffraction pattern of BaY2ZnO5 was used for performing phase identification. While the La, Nd, and Y preparations were phase-pure, small concentrations of binary oxides were observed in the Eu, Dy, Ho, and Er products. A minor concentration of an unidentified phase was detected in the Sm, Eu, and Gd products. The Tm preparation contained significant concentrations of Ba5Zn4Tm8O21 [13,14] and Tm2O3 (see Fig. 1a). The Yb and Lu preparations yielded only Ba5Zn4R8O21 and R2O3.
Fig. 1. X-ray diffraction patterns of (a) BaTm2ZnO5 and (b) BaSm2ZnO5. The impurity phases are marked.
For the Rietveld refinements, the powders were mounted in zero-background quartz holders with double-sided adhesive tape. A Scintag PAD V diffractometer1 equipped with an Ortec intrinsic Ge detector was used to measure the powder patterns (Cu Kα radiation, 40 kV, 30 mA) for values of 2θ from 3° – 140° in 0.02° steps, counting after each step for 10 s or 12 s.
All data processing was carried out using the GSAS software suite [12]. To minimize the effects of surface roughness and incomplete interception of the beam, only the 18 ° to 140° portions of the patterns were used in the refinements. The initial structure models were taken from Ref. [9]. For the La and Nd compounds, the tetragonal space group I4/mcm was used, while for compounds of the smaller lanthanides, the orthorhombic space group Pbnm (an alternate setting of Pnma) was used.
All atomic positions were refined isotropically. The displacement coefficients of the two independent lanthanide ions in Pbnm were constrained to have a common value. In all refinements, a single isotropic displacement coefficient was refined for the oxygen atoms. A scale factor and the lattice parameters were refined for the major phase. In some samples, impurity phases were detected, and were subsequently included in the refinements using fixed structural models. The peak profiles were described using a pseudo-Voigt function. Only the Cauchy X, asymmetry, and sample displacement parameters were refined. Backgrounds were described using a 3-term cosine Fourier series.
-
1Certain commercial equipment, instruments, or materials are identified in this paper to foster understanding. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
3. Results and Discussion
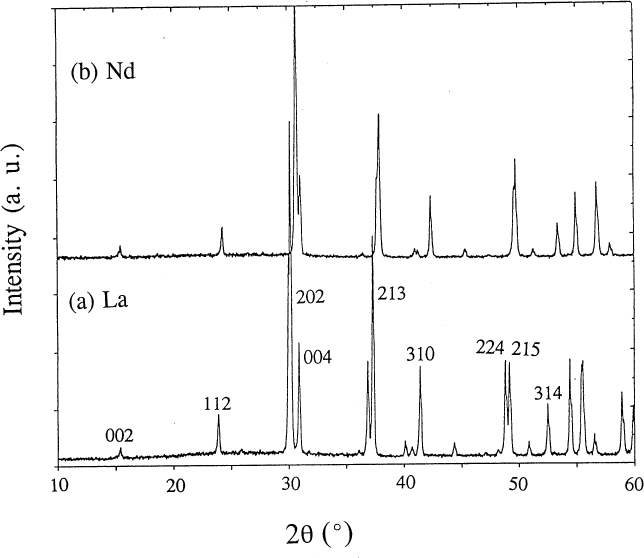
As the ionic size of R in BaR2ZnO5 decreases, it becomes progressively more difficult to prepare the BaR2ZnO5 phase. This phase cannot be prepared for R=Yb and Lu under the specified conditions. We believe that under more appropriate heat treatment conditions (i.e., different oxygen partial pressure), a single phase can be formed in the Ba-Tm-Zn system. This possibility is under investigation. Since the refined structural parameters for BaTm2ZnO5 are not as accurate or precise as those derived from the pattern of a pure phase, these parameters will not be discussed. The x-ray diffraction pattern of the nominal single phase BaSm2ZnO5 is illustrated in Fig. 1b; selected Miller indices are indicated. Figures 2a and 2b show the x-ray diffraction patterns of BaLa2ZnO5 and BaNd2ZnO5. The patterns of these analogs are similar. The small displacement of the corresponding peaks in the La and Nd patterns indicates the effect of ionic size on the isostructural compounds.
Fig. 2. X-ray diffraction patterns of (a) BaLa2ZnO5 and (b) BaNd2ZnO5.
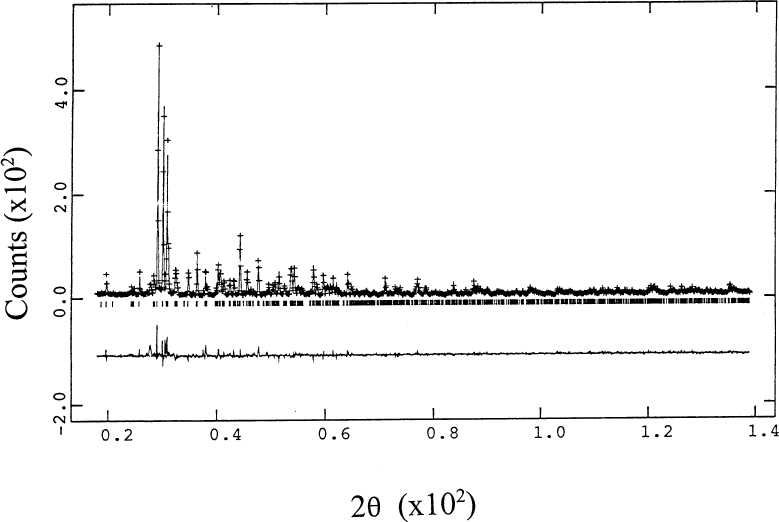
The refinement residuals using the GSAS suite [12] are reported in Table 2. The observed, calculated, and difference pattern of BaSm2ZnO5 is illustrated in Fig. 3. The upper graph shows the fit between the experimental and calculated patterns while the lower one shows the difference between these two patterns. The refined structural parameters for the tetragonal compounds are reported in Table 3, and those of the orthorhombic phases are reported in Table 4. Global parameters are given in Table 5. Selected structural quantities such as bond lengths and bond angles are reported in Tables 6 and 7. Also reported in the above tables are results of the neutron refinements of the lattice parameters [9] and bond lengths and angles for the R=La, Nd, Dy, Ho, Y, and Er compounds for comparison wherever appropriate. Although the refined overall structure, structural parameters, and bond distances derived from the x-ray and neutron refinements [9] for the tetragonal structures agree quite well, there are differences in the bond distances for some of the orthorhombic structures. These small differences are most likely associated with the relatively large uncertainties in the determination of the oxygen positions by using x-ray techniques.
Table 2. Rietveld refinement residuals for BaR2ZnO5using the GSAS software system. The ionic radius of R (R+3) used are 8-fold coordinated for R=La, and Nd and 7-fold coordinated for the rest of the smaller ions [16]. The values for the Ho and Tm analogs were estimated by using the interpolated values between those of the 6- and 8-fold coordination.
| R | La | Nd | Sm | Eu | Gd | Dy | Ho | Y | Er |
| R3+(Å) | 1.160 | 1.109 | 1.02 | 1.01 | 1.00 | 0.97 | 0.96 | 0.96 | 0.945 |
| wRp | 0.0997 | 0.1339 | 0.1486 | 0.1184 | 0.0811 | 0.0733 | 0.0734 | 0.1145 | 0.0712 |
| Rp | 0.0749 | 0.0899 | 0.1107 | 0.0893 | 0.0627 | 0.0577 | 0.0568 | 0.0853 | 0.0524 |
| R(F) | 0.0593 | 0.0436 | 0.0592 | 0.0536 | 0.0374 | 0.0493 | 0.0353 | 0.0408 | 0.0369 |
| χ2 | 1.756 | 2.355 | 2.912 | 1.927 | 1.431 | 1.703 | 1.883 | 3.918 | 2.463 |
| No. var. | 15 | 15 | 30 | 35 | 30 | 33 | 32 | 30 | 33 |
| ΔF +/- | 4.4/-3.6 | 4.1/-2.2 | 3.6/-2.7 | 4.9/-2.8 | 3.0/-2.0 | 2.5/-2.5 | 4.4/-1.8 | 1.6/-2.2 | 1.8/-1.9 |
Table 3. Refined Structural Parameters of tetragonal BaR2ZnO5 (Space Group I4/mcm ). Values in italics are from the neutron refinements. The numbers inside the parenthesis are standard uncertainties. Z is the number of formulas per unit cell. Uiso is the isotropic temperature factor.
| R | La | Nd |
| Cell parameter, a ( Å) |
6.90991(7) 6.9118(1) |
6.75982(4) 6.7608(1) |
| c (Å) |
11.59782(16) 11.6002(2) |
11.54565(11) 11.5442(2) |
| V(Å3) | 553.759 | 527.580 |
| Ba, 00¼ , Uiso (Å2) |
0.0048(4) 0.0098(5) |
0.0042(4) 0.0105(5) |
| R, x (½+x)0, x |
0.1737(1) 0.1743(1) |
0.1743(1) 0.1744(1) |
| Uiso (Å2) |
0.0030(3) 0.0073(2) |
0.0024(2) 0.0066(3) |
| Zn, 0½¼, Uiso (Å2) |
0.0115(9) 0.0049(4) |
0.0056(7) 0.0091(5) |
| O1, 000 , Uiso (Å2) |
0.0039(18) 0.0106(5) |
0.0044(16) 0.0121(6) |
| O2, x (½+x )z, x |
0.3548(9) 0.3553(1) |
0.3564(8) 0.3547(1) |
| z |
0.1357(6) 0.1337(1) |
0.1310(6) 0.1314(1) |
| Uiso (Å2) |
0.0039(18) 0.0115(3) |
0.0044(16) 0.0118(3) |
Table 4. Refined Structural Parameters of the Orthorhombic BaR2ZnO5compounds (Space Group Pbnm).Values in italics are from neutron/synchrotron (Er-analog) refinements. The numbers inside the parenthesis are standard uncertainties. Z is the number of formulas per unit cell. Uiso is the isotropic temperature factor.
| R | Sm | Eu | Gd | Dy | Ho | Y | Er | Tm |
| Cell, a (Å) | 7.20447(14) | 7.17890(10) | 7.15729(9) |
7.09330(7) 7.0944(1) |
7.07113(6) 7.0713(1) |
7.07018(7) 7.0707(1) |
7.04515(7) 7.0472(1) |
7.01855(9) |
| b(Å) | 12.58817(23) | 12.53575(17) | 12.49393(15) |
12.38464(13) 12.3885(2) |
12.34199(11) 12.3437(2) |
12.33678(12) 12.3386(1) |
12.29815(12) 12.3022(1) |
12.25445(17) |
| c (Å) | 5.81214(10) | 5.79103(8) | 5.77424(7) |
5.72979(6) 5.7314(1) |
5.71158(5) 5.7120(2) |
5.70908(5) 5.7095(1) |
5.69410(5) 5.6958(1) |
5.67786(14) |
| V (Å3) | 527.109 | 521.152 | 516.348 | 503.350 | 498.460 | 497.964 | 493.350 | 488.344 |
| Ba, xy 1/4, x | 0.9246(3) | 0.9238(3) | 0.9241(2) |
0.9240(3) 0.9252(10) |
0.9238(2) 0.9228(5) |
0.9238(2) 0.9231(4) |
0.9239(2) 0.9229(2) |
0.9235(4) |
| y | 0.9012(2) | 0.9009(2) | 0.9006(1) |
0.8999(2) 0.9007(5) |
0.8994(1) 0.9001(3) |
0.8998(1) 0.9001(2) |
0.8989(1) 0.8995(1) |
0.8999(2) |
| Uiso, Å2 | 0.0071(7) | 0.0059(6) | 0.0062(5) |
0.0039(6) 0.020(2) |
-0.0001(4) 0.0103(9) |
0.0039(4) 0.0093(6) |
0.0049(5) 0.0059(3) |
0.0015(20) |
| R1, xy 1/4, x | 0.1186(3) | 0.1182(3) | 0.1196(3) |
0.1206(3) 0.1210(4) |
0.1202(2) 0.1206(3) |
0.1204(3) 0.1201(2) |
0.1205(2) 0.1209(2) |
0.1208(5) |
| y | 0.2920(2) | 0.2925(2) | 0.2930(1) |
0.2926(2) 0.2927(2) |
0.2924(1) 0.2918(2) |
0.2919(2) 0.2919(1) |
0.2924(1) 0.2923(1) |
0.2935(2) |
| Uiso, Å2 | 0.0041(4) | 0.0030(3) | 0.0017(3) |
0.0020(3) 0.0170(6) |
0.0096(3) 0.0047(5) |
0.0018(3) 0.0055(3) |
0.0022(3) 0.0033(2) |
0.002 |
| R2, xy 1/4, x | 0.3965(3) | 0.3977(3) | 0.3977(2) |
0.3992(3) 0.3984(3) |
0.3997(2) 0.3988(3) |
0.3988(3) 0.3989(2) |
0.4001(2) 0.3994(1) |
0.3963(4) |
| y | 0.0750(2) | 0.0747(2) | 0.0745(1) |
0.0740(2) 0.0744(2) |
0.0746(1) 0.0741(2) |
0.0735(2) 0.0739(1) |
0.0744(1) 0.0743(1) |
0.0722(2) |
| Uiso, Å2 | 0.0041(4) | 0.0030(3) | 0.0017(3) |
0.0020(3) 0.0157(5) |
0.0096(3) 0.0043(5) |
0.0018(3) 0.0063(3) |
0.0022(3) 0.0028(2) |
0.002 |
| Zn, xy 1/4, x | 0.6890(7) | 0.6908(6) | 0.6909(5) |
0.6920(6) 0.6984(9) |
0.6936(4) 0.6904(4) |
0.6910(4) 0.6907(3) |
0.6927(5) 0.6902(3) |
0.6908(9) |
| y | 0.6499(4) | 0.6497(4) | 0.6490(3) |
0.6502(3) 0.6492(6) |
0.6501(2) 0.6505(3) |
0.6499(2) 0.6501(2) |
0.6493(3) 0.6501(2) |
0.6555(6) |
| Uiso, Å2 | 0.0071(16) | 0.0076(14) | 0.0046(12) |
0.0059(12) 0.0179(13) |
-0.0022(8) 0.0074(6) |
0.0073(8) 0.0061(4) |
0.0041(10) 0.0035(5) |
0.0011(20) |
| O1, x | 0.1692(22) | 0.1662(18) | 0.1657(15) |
0.1754(16) 0.1678(6) |
0.1645(12) 0.1669(3) |
0.1578(12) 0.1663(2) |
0.1744(14) 0.1666(13) |
0.1720(26) |
| y | 0.4292(17) | 0.4368(14) | 0.4323(11) |
0.4333(13) 0.4343(5) |
0.4315(9) 0.4347(2) |
0.4323(8) 0.4344(1) |
0.4312(11) 0.4323(11) |
0.4231(25) |
| z | -0.0226(29) | -0.0052(23) | -0.0018(19) |
-0.0083(21) -0.0008(8) |
-0.0085(16) -0.0036(4) |
-0.0036(16) -0.0024(3) |
-0.0050(18) 0.000(2) |
-0.026(4) |
| Uiso, Å2 | 0.014(3) | 0.006(2) | 0.004(2) |
0.005(2) 0.019(1) |
0.01 0.0093(4) |
0.0031(14) 0.0074(3) |
0.003(2) 0.010(2) |
0.012(4) |
| O2, x | 0.3566(26) | 0.3530(21) | 0.3547(18) |
0.3554(20) 0.3586(8) |
0.3574(15) 0.3592(3) |
0.3503(13) 0.3586(2) |
0.3607(17) 0.3579(15) |
0.367(4) |
| y | 0.2174(15) | 0.2216(12) | 0.2225(10) |
0.2261(11) 0.2249(4) |
0.2261(8) 0.2249(2) |
0.2281(7) 0.2251(1) |
0.2250(9) 0.2240(8) |
0.2310(19) |
| z | 0.5020(31) | 0.5025(25) | 0.5023(21) |
0.4899(22) 0.5058(10) |
0.5015(17) 0.5020(5) |
0.5054(18) 0.5014(3) |
0.5030(19) 0.5045(2) |
0.499(5) |
| Uiso, Å2 | 0.014(3) | 0.006(2) | 0.004(2) |
0.005(2) 0.022(1) |
0.01 0.0097(5) |
0.0031(14) 0.0093(3) |
0.003(2) 0.006(2) |
0.012(4) |
| O3, xy 1/4, x | 0.0663(34) | 0.0833(29) | 0.0748(24) |
0.0755(26) 0.0762(11) |
0.0690(19) 0.0763(5) |
0.0817(20) 0.0752(3) |
0.0776(22) 0.0756(2) |
0.110(5) |
| y | 0.0996(21) | 0.0978(17) | 0.0991(14) |
0.0954(16) 0.0999(6) |
0.1004(12) 0.1005(3) |
0.0986(11) 0.1008(2) |
0.0997(13) 0.1024(11) |
0.0971(25) |
| Uiso, Å2 | 0.014(3) | 0.006(2) | 0.004(2) |
0.005(2) 0.026(2) |
0.01 0.0100(8) |
0.0031(14) 0.0077(5) |
0.003(2) 0.006(3) |
0.012(4) |
Table 5. Refined global parameters of BaR2ZnO5using the GSAS software suite [12]. The meaning of the variables listed in this table are given in Ref. [12]. The numbers inside the parenthesis are standard uncertainties. The impurity phases are expressed in mass fraction %.
| R | La | Nd | Sm | Eu | Gd | Dy | Ho | Y | Er | Tm |
| Second Phases | unident |
3 % Eu2O3 0.5 % ZnO unident. |
unident. | 3 % Dy2O3 | 2 % Ho2O3 | 5 % Er2O3 |
11% Tm2O3 43% Ba5Zn4Tm8O21 |
|||
| Profile X | 3.63(8) | 1.60(5) | 2.78(5) | 0.60(5) | 0.88(4) | 0.49(4) | 0.98(4) | 0.26(12) | ||
| Y | 12.7(1) | 8.2(1) | ||||||||
| asym | 3.6(2) | 1.9(2) | 3.51(1) | 3.5(1) | 3.2(1) | 2.3(1) | 3.5(1) | 2.1(1) | ||
| BK1 | 10.05(3) | 5.40(3) | 6.20(4) | 7.48(3) | 13.90(4) | 22.72(5) | 21.20(5) | 11.27(6) | 31.25(7) | 24.79(8) |
| BK2 | 1.39(4) | 0.62(4) | 0.58(5) | 70.06(4) | 0.02(5) | 0.19(7) | 0.24(7) | 0.21(7) | 0.50(9) | -2.28(11) |
| BK3 | 4.76(4) | 2.38(4) | 2.20(4) | 1.88(4) | 1.96(4) | 2.46(6) | 1.75(6) | 1.79(6) | 2.29(8) | 1.67(10) |
Table 6. Selected bond distances (Å) in tetragonal BaR2ZnO5compounds. Distances in italics are from the neutron refinements.
| R | La | Nd |
| Ba-O1×2 |
2.89945(4) 2.90004(4) |
2.88641(3) 2.88605(4) |
| Ba-O2×8 |
2.962(4) 2.9749(6) |
2.939(4) 2.9308(7) |
| R-O1×2 |
2.5542(4) 2.5532(3) |
2.4970(3) 2.4971(3) |
| R-O2×2 |
2.369(9) 2.3531(14) |
2.306(8) 2.2964(15) |
| R-O2×4 |
2.715(6) 2.7038(11) |
2.636(6) 2.6483(12) |
| Zn-O2×4 |
1.941(9) 1.9541(11) |
1.942(8) 1.9501(12) |
Table 7. Selected bond distances (Å) in Orthorhombic BaR2ZnO5compounds. Values in italics are from the neutron/synchrotron (Er-analog) refinements.
| R | Sm | Eu | Gd | Dy | Ho | Y | Er |
| Ba-O1×2 | 3.346(17) | 3.324(14) | 3.300(11) |
3.231(12) 3.252(8) |
3.288(9) 3.271(4) |
3.318(9) 3.269(3) |
3.205(10) 3.249(10) |
| Ba-O1'×2 | 3.113(17) | 3.093(14) | 3.136(11) |
3.049(13) 3.091(9) |
3.101(9) 3.069(4) |
3.132(9) 3.076(3) |
3.067(11) 3.094(11) |
| Ba-O2×2 | 3.159(19) | 3.124(15) | 3.094(12) |
2.995(13) 3.041(8) |
3.005(10) 3.271(4) |
3.027(9) 3.269(3) |
2.992(11) 3.286(11) |
| Ba-O2'×2 | 2.900(18) | 2.891(15) | 2.897(12) |
2.929(14) 2.904(9) |
2.893(10) 3.020(4) |
2.862(10) 3.018(2) |
2.885(11) 3.025(10) |
| Ba-O3 | 2.699(28) | 2.722(22) | 2.705(19) |
2.650(20) 2.689(10) |
2.685(15) 2.702(4) |
2.695(14) 2.699(3) |
2.696(17) 2.718(14) |
| Ba-O3'×2 | 2.9068(6) | 2.8960(4) | 2.88713(6) |
2.8655(4) 2.866(7) |
2.8562(2) 2.856(5) |
2.8549(2) 2.855(4) |
2.8471(1) 2.848(13) |
| R1-O1×2 | 2.372(19) | 2.361(15) | 2.293(13) |
2.319(14) 2.292(6) |
2.285(10) 2.271(3) |
2.273(9) 2.297(2) |
2.273(11) 2.258(11) |
| R1-O2×2 | 2.443(18) | 2.402(15) | 2.394(13) |
2.411(14) 2.387(6) |
2.355(10) 2.364(7) |
2.273(9) 2.363(2) |
2.372(11) 2.365(10) |
| R1-O2'×2 | 2.378(19) | 2.389(15) | 2.382(13) |
2.311(14) 2.339(6) |
2.350(10) 2.370(3) |
2.321(10) 2.341(2) |
2.318(12) 2.331(10) |
| R1-O3 | 2.450(27) | 2.453(21) | 2.443(18) |
2.463(20) 2.409(8) |
2.397(15) 2.304(4) |
2.400(13) 2.379(2) |
2.388(16) 2.358(14) |
| R2-O1×2 | 2.471(21) | 2.398(13) | 2.396(11) |
2.401(12) 2.302(7) |
2.347(11) 2.306(3) |
2.311(9) 2.291(2) |
2.384(10) 2.301(12) |
| R2-O1×2 | 2.369(16) | 2.320(16) | 2.340(14) |
2.347(15) 2.388(5) |
2.327(9) 2.367(3) |
2.300(10) 2.263(2) |
2.342(12) 2.363(10) |
| R2-O2×2 | 2.333(19) | 2.373(15) | 2.374(13) |
2.352(14) 2.389(6) |
2.377(10) 2.338(3) |
2.425(10) 2.371(2) |
2.363(11) 2.362(10) |
| R2-O3 | 2.399(25) | 2.275(21) | 2.331(18) |
2.312(18) 2.308(8) |
2.360(14) 2.381(4) |
2.263(14) 2.313(3) |
2.294(15) 2.308(14) |
| Zn-O1×2 | 1.944(19) | 2.059(15) | 2.035(13) |
1.967(14) 2.034(7) |
1.980(10) 2.026(3) |
2.037(10) 2.027(2) |
1.950(11) 2.019(11) |
| Zn-O2×2 | 2.230(19) | 2.181(15) | 2.174(13) |
2.164(13) 2.123(8) |
2.116(10) 2.120(4) |
2.074(10) 2.124(2) |
2.124(11) 2.114(10) |
| Zn-O3 | 1.945(24) | 2.072(20) | 2.002(17) |
2.015(17) 1.981(10) |
1.956(13) 1.972(10) |
2.029(13) 1.976(3) |
1.999(15) 1.963(14) |
Fig. 3. Rietveld refinement results for BaSm2ZnO5. The upper graph shows the fit between the experimental and calculated patterns while the lower graph shows the difference between these two patterns.
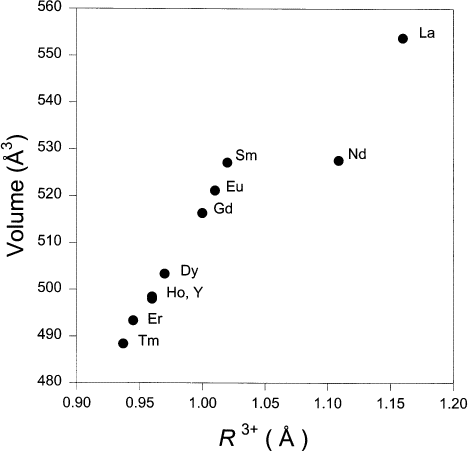
The trend of unit cell volume with the ionic radius, R3 + of BaR2ZnO5 (where R 3+=La, Nd, Sm, Eu, Gd, Dy, Ho, Y, Er, and Tm) is illustrated in Fig. 4. These ionic radii are chosen based on the structural environment surrounding these ions, namely, 8-fold coordination for R=La and Nd, and 7-fold coordination for the rest of the smaller ions [15,16]. Since the 7-fold coordination radii for the Ho and Tm analogs were not reported, they were estimated by interpolating between those for 6- and 8-fold coordination. A monotonic decrease of the volumes vs the ionic radius from Sm to Tm is observed, agreeing well with the trend expected from lanthanide contraction. Since the La and Nd analogs adopt a different structure, the cell parameters of these two compounds do not fall on the same line as the smaller analogs.
Figure 4. Plot of unit cell volume vs R 3+ (R=La, Nd, Sm, Eu, Gd, Dy, Y, Ho, Er and Tm) as found in BaR2ZnO5. R3+ is the ionic radius for coordination number (C.N.)=8 for the La and Nd compounds and C.N.=7 for the rest [15,16].
3. 1. Crystal Structures of BaR2ZnO5
It has been reported that, since the effective ionic radii of Zn2+ (0.68 Å for coordination number (C.N.) of 5, and 0.60 Å for C.N.=4) and Cu2+ (0.65 Å for C.N.= 5, and 0.57 Å for C.N.=4) are comparable [15,16], it is possible for the Zn series and the Cu series (BaR2CuO5) to be isostructural. Results of both neutron and x-ray Rietveld refinements showed that the BaR2ZnO5 compounds with smaller R are indeed isostructural to the “green phase” compounds [6,8,9,17]. When R=La and Nd, the structures of BaR2ZnO5 are different from the “brown phase” BaR2CuO5 analogs. The refined atomic coordinates for the La and Nd compounds agree well with those reported by Michel et al. using x-ray diffraction [6,8] and Wong-Ng et al. [9].
3. 1. 1. Structure of Tetragonal BaR2ZnO5, R=La or Nd
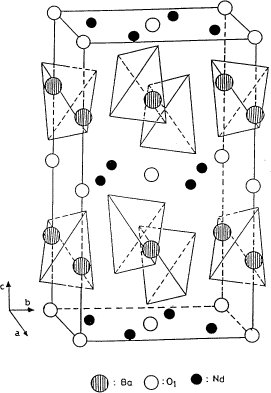
Detailed descriptions of the structures of BaLa2ZnO5 and BaNd2ZnO5 have been reported by Michel et al. [6,7], Taibi et al. [18], and Wong-Ng et al. [9] (Fig. 5). Despite similar effective ionic sizes of Zn2+ and Cu2+, the structures of BaR2ZnO5 and the brown phase BaR2 CuO5 compounds are different [5,19]. The brown phase crystallizes in the space group P4/mbm, and the structure contains square planar CuO4 groups. The Zn analogs consist of a three-dimensional array of interconnected BaO10 and RO8 polyhedra as well as tetrahedral ZnO4 groups (see Fig. 5). The RO8 polyhedron is a trigonal prism capped on two of the three rectangular faces, and the capped BaO10 polyhedron is a square prism capped on both ends by tetragonal pyramids. The structure can be viewed as consisting of alternate layers of Zn-Ba-O and R-O extending infinitely in the abplane and perpendicular to the c axis.
Figure 5. Structure of BaNd2ZnO5, showing the ZnO4 tetrahedra. Ba ions are located between these tetrahedra.
The atomic valence values Vb for Ba, R/R1, R2, and Zn were calculated [20] and are listed in Table 8. The Vb of an atom i is defined as the sum of the bond valences vij of all the bonds from atom i to atoms j (Vbi = Σ vij). The most commonly adopted empirical expression for the bond valence vij as a function of the interatomic distance dij is
vij = exp [(r0 − dij)/B].
The parameter B is commonly taken to be a “universal” constant equal to 0.37 Å [20,21]. The values of the reference distance r 0 are tabulated for various pairs of atoms [20]. The Vb values (1.686 and 1.784) of Ba in the La and Nd analogs indicate an underbonded situation in both compounds. These values, which agree with those derived from neutron data, are significantly less than the expected value of 2 and indicate the size of the BaO10 cages are relatively large. The Vb values for Zn (2.108 and 2.102 in the La- and Nd-analogs) are very close to the expected value of 2.
Table 8. Atomic valence values (Vb) for the Ba, Zn and R calculated form the measured bond distances, A [20, 21]. R1 and R2 are used for the two independent lanthanide sites in the BaR2ZnO5compounds containing Sm, Eu, Gd, Dy, Ho, Y and Er. The values initalicsrefer to calculations based on data from neutron/synchrotron (Er-analog) refinements [9].
| R | Ba | R or R1 | R2 | Zn |
| La |
1.686 1.641 |
3.269 2.890 |
- |
2.108 2.035 |
| Nd |
1.784 1.815 |
2.900 2.899 |
- |
2.102 2.057 |
| Sm | 1.616 | 3.005 | 3.109 | 2.049 |
| Eu | 1.654 | 2.974 | 3.352 | 1.687 |
| Gd | 1.673 | 3.111 | 3.124 | 1.826 |
| Dy |
1.868 1.783 |
2.923 3.023 |
2.934 2.997 |
1.991 1.937 |
| Ho |
1.817 1.565 |
2.991 3.070 |
2.883 2.954 |
2.001 1.972 |
| Y |
1.798 1.568 |
3.211 2.909 |
2.988 3.174 |
1.964 1.958 |
| Er |
1.892 1.544 |
2.964 3.020 |
2.778 2.901 |
2.122 2.011 |
3. 1. 2. Structure of Orthorhombic BaRZnO5, R= Sm, Eu, Gd, Dy, Ho, Y, or Er2
The structures of the orthorhombic BaR2ZnO5 compounds are similar to those of the “green phase” BaR2CuO5 analogs. The detailed structure of the green phase type structure has been reported [6,7,9,22,23,24]. The basic structure of these compounds consists of RO7, BaO11, and ZnO5 polyhedra. R is 7-fold coordinated inside a monocapped trigonal prism, and two such units join to form the basic structure motif of R2O11. The Ba atoms are found to reside in distorted 11-fold coordinated cages, characterized by Ba – O distances between 2.650(20) Å and 3.346(17) Å , where the values inside the brackets are standard uncertainties. Both CuO5 and ZnO5 in BaR2CuO5 and BaR2ZnO5 have a distorted tetragonal pyramidal coordination. Similar to the La-analog, the Vb values for Ba are less than 2 (Table 8). The Zn – O distances in the seven compounds range from 1.950(11) Å to 2.164(13) Å . The Vb values for R and Zn do not deviate significantly from the expected values of 3 and 2, respectively, except for the Eu-analog. In this compound, the Eu2-O polyhedron appears to be relatively small (overbonded, with Vb =3.353), whereas the size of the Zn-O square pyramid is relatively large (Vb= 1.687). This result may be due to the relatively large uncertainty associated with the position of O3 derived from the x-ray data.
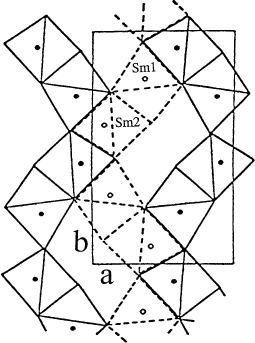
In Fig. 6, the projection of the Sm2O11 blocks at z= 1/4 is shown as solid lines and the second layer at z=3/4 is represented as dotted lines. These prisms share edges to form wave-like chains parallel to the long b-axis. Chains are crosslinked by Cu and Ba atoms. The c direction is the shortest axis, and is also the direction in which layers of prisms are stacked parallel to each other, sharing the trigonal faces.
Figure 6. Projection of the structure of BaSm2ZnO5 in the (001) plane showing the linkage of R2O11 polyhedra at z =1/4 (broken line) and z=3/4 (solid line). The wave-like chains along the b-axis are displayed, and the two independent Sm atoms are labelled.
3. 2. Reference X-Ray Diffraction Patterns
Reference x-ray powder patterns of the nine compounds BaR2ZnO5, in which R=La, Nd, Sm, Eu, Gd, Dy, Ho, Y, or Er, were obtained using a pattern decomposition technique. Because the refined structural parameters for the R=Tm analog are not as accurate or precise as those derived from the pattern of a pure or nearly pure phase, the x-ray diffraction pattern of this phase is not reported here. These patterns represent ideal specimen patterns. They are corrected for systematic errors both in dand I. The reported peak positions are calculated from the refined lattice parameters, as this represents the best measure of the true positions. For peaks resolved at the instrument resolution function, the individual peak positions are reported. For overlapping peaks, the intensity weighted average peak position is reported with multiple indices. For marginally resolved peaks, individual peaks are reported, to more accurately simulate the visual appearance of the pattern.
Tables 9 to 17 list these patterns with d spacings, Miller indices h, k, l and integrated intensities I, normalized to the value 999 as the maximum. The symbols M and + refer to peaks containing contributions from two and more than two reflections, respectively. These patterns have been submitted to International Centre for Diffraction Data (ICDD) for inclusion in the PDF.
4. Summary
The reference x-ray diffraction patterns and the crystal structures of both tetragonal and orthorhombic BaR2ZnO5, R=La, Nd, Sm, Eu, Gd, Dy, Ho, Y, or Er were obtained by Rietveld refinement. The most striking difference between the orthorhombic and tetragonal BaR2ZnO5 structures is the Zn-O coordination environment. In the orthorhombic structure, the Zn atom is coordinated to five oxygen atoms, four of which form the base of a square pyramid, whereas in the tetragonal structure, the Zn atom is tetrahedrally coordinated.
Table 9. X-ray diffraction pattern of BaLa2ZnO5 with d spacings (Å), Miller indices h k l, and integrated intensities I normalized to the value of 999 as the maximum.
| d | I | h | k | l | d | I | h | k | l | d | I | h | k | l |
| 3.79885 | 53 | 0 | 0 | 2 | 3.73647 | 200 | 1 | 1 | 2 | 3.45491 | 20 | 2 | 0 | 0 |
| 2.98599 | 480 | 2 | 1 | 1 | 2.96806 | 999 | 2 | 0 | 2 | 2.89942 | 345 | 0 | 0 | 4 |
| 2.49345 | 22 | 1 | 1 | 4 | 2.44299 | 239 | 2 | 2 | 0 | 2.41379 | 540 | 2 | 1 | 3 |
| 2.25136 | 39 | 2 | 2 | 2 | 2.22096 | 20 | 2 | 0 | 4 | 2.18508 | 208 | 3 | 1 | 0 |
| 2.04473 | 31 | 3 | 1 | 2 | 1.93295 | 43 | 0 | 0 | 6 | 1.89080 | 18 | 3 | 2 | 1 |
| 1.86824 | 214 | 2 | 2 | 4 | 1.85508 | 207 | 2 | 1 | 5 | 1.79741 | 34 | 1 | 1 | 6 |
| 1.74502 | 114 | 3 | 1 | 4 | 1.72746 | 7 | 4 | 0 | 0 | 1.68688 | 178 | 2 | 0 | 6 |
| 1.65865 | 135 | 4 | 1 | 1 | 1.65556 | 104 | 4 | 0 | 2 | 1.62866 | 33 | 3 | 3 | 0 |
| 1.56799 | 97 | 3 | 3 | 2 | 1.54508 | 77 | 4 | 2 | 0 | 1.53762 | 131 | 4 | 1 | 3 |
| 1.49299 | 21 | 4 | 2 | 2 | 1.48403 | 19 | 4 | 0 | 4 | 1.46018 | 37 | 2 | 1 | 7 |
| 1.44971 | 44 | 0 | 0 | 8 | 1.44777 | 8 | 3 | 1 | 6 | 1.36356 | 82 | 4 | 2 | 4 |
| 1.35842 | 76 | 4 | 1 | 5 | 1.31957 | 27 | 5 | 1 | 2 | 1.28804 | 53 | 4 | 0 | 6 |
| 1.27534 | 24 | 5 | 2 | 1 | 1.24672 | 53 | 2 | 2 | 8 | 1.24549 | 66 | 3 | 3 | 6 |
| 1.22150 | 20 | 4 | 4 | 0 | 1.21780 | 41 | 5 | 2 | 3 | 1.20775 | 63 | 3 | 1 | 8M |
| 1.20775 | 63 | 4 | 2 | 6M | 1.19527 | 12 | 4 | 4 | 2 | 1.18936 | 36 | 2 | 1 | 9 |
| 1.18502 | 32 | 5 | 3 | 0 | 1.17823 | 42 | 4 | 1 | 7 | 1.16103 | 18 | 5 | 3 | 2 |
| 1.15164 | 56 | 6 | 0 | 0 | 1.13032 | 7 | 6 | 1 | 1+ | 1.12881 | 5 | 1 | 1 | 10+ |
| 1.12568 | 16 | 4 | 4 | 4 | 1.12278 | 27 | 5 | 2 | 5 | 1.10961 | 16 | 5 | 1 | 6 |
| 1.09948 | 42 | 2 | 0 | 10 | 1.09694 | 58 | 5 | 3 | 4 | 1.08286 | 17 | 3 | 3 | 8 |
| 1.07383 | 102 | 5 | 4 | 1M | 1.07383 | 102 | 6 | 2 | 2M | 1.07030 | 69 | 6 | 0 | 4 |
| 1.05721 | 45 | 4 | 2 | 8 | 1.03940 | 31 | 5 | 4 | 3 | 1.02155 | 32 | 4 | 1 | 9 |
| 1.01447 | 11 | 5 | 2 | 7 | 1.01028 | 8 | 5 | 3 | 6 | 0.99785 | 30 | 2 | 1 | 11 |
| 0.97843 | 24 | 5 | 4 | 5 | 0.96647 | 13 | 0 | 0 | 12 | 0.96361 | 14 | 5 | 5 | 2M |
| 0.96361 | 14 | 7 | 1 | 2M | 0.96289 | 18 | 4 | 0 | 10 | 0.95112 | 64 | 6 | 2 | 6 |
| 0.94564 | 70 | 7 | 2 | 1M | 0.94564 | 70 | 6 | 4 | 2M | 0.94472 | 30 | 3 | 3 | 10 |
| 0.93412 | 15 | 4 | 4 | 8 | 0.92176 | 30 | 7 | 2 | 3 | 0.91750 | 52 | 5 | 3 | 8 |
| 0.90951 | 31 | 6 | 4 | 4M | 0.90951 | 31 | 5 | 2 | 9M | 0.90424 | 20 | 5 | 4 | 7 |
| 0.90174 | 74 | 6 | 0 | 8 | 0.89870 | 25 | 2 | 2 | 12 | 0.89242 | 45 | 4 | 1 | 11 |
| 0.88388 | 28 | 3 | 1 | 12 | 0.88113 | 16 | 5 | 1 | 10 | 0.87844 | 32 | 7 | 2 | 5 |
| 0.87209 | 20 | 7 | 1 | 6M | 0.87209 | 20 | 5 | 5 | 6M | 0.86590 | 7 | 7 | 3 | 4 |
| 0.85852 | 46 | 6 | 4 | 6 | 0.85713 | 26 | 2 | 1 | 13 | 0.85452 | 100 | 8 | 1 | 1M |
| 0.85452 | 100 | 7 | 4 | 1M | 0.85452 | 100 | 8 | 0 | 2M | 0.83794 | 31 | 8 | 2 | 0 |
| 0.83674 | 70 | 7 | 4 | 3M | 0.83674 | 70 | 8 | 1 | 3M | 0.82887 | 6 | 5 | 3 | 10 |
| 0.82735 | 16 | 5 | 4 | 9 | 0.82357 | 27 | 7 | 2 | 7 |
Table 10. X-ray diffraction pattern of BaNd2ZnO5 withd spacings (Å), Miller indices h k l, and integrated intensitiesI normalized to the value of 999 as the maximum.
| d | I | h | k | l | d | I | h | k | l | d | I | h | k | l |
| 5.77280 | 93 | 0 | 0 | 2 | 4.77989 | 8 | 1 | 1 | 0 | 3.68165 | 169 | 1 | 1 | 2 |
| 3.37989 | 20 | 2 | 0 | 0 | 2.92448 | 484 | 2 | 1 | 1 | 2.91674 | 999 | 2 | 0 | 2 |
| 2.88640 | 340 | 0 | 0 | 4 | 2.47085 | 31 | 1 | 1 | 4 | 2.38995 | 234 | 2 | 2 | 0 |
| 2.37733 | 541 | 2 | 1 | 3 | 2.20819 | 29 | 2 | 2 | 2 | 2.19493 | 17 | 2 | 0 | 4 |
| 2.13763 | 197 | 3 | 1 | 0 | 2.00461 | 18 | 3 | 1 | 2 | 1.92427 | 5 | 0 | 0 | 6 |
| 1.85059 | 9 | 3 | 2 | 1 | 1.84082 | 184 | 2 | 2 | 4 | 1.83504 | 206 | 2 | 1 | 5 |
| 1.78505 | 25 | 1 | 1 | 6 | 1.71784 | 104 | 3 | 1 | 4 | 1.68995 | 5 | 4 | 0 | 0 |
| 1.67224 | 171 | 2 | 0 | 6 | 1.62265 | 239 | 4 | 1 | 1M | 1.62265 | 239 | 4 | 0 | 2M |
| 1.59330 | 38 | 3 | 3 | 0 | 1.53587 | 121 | 3 | 3 | 2 | 1.51153 | 81 | 4 | 2 | 0 |
| 1.50833 | 141 | 4 | 1 | 3 | 1.49883 | 7 | 2 | 2 | 6 | 1.46224 | 29 | 4 | 2 | 2 |
| 1.45837 | 23 | 4 | 0 | 4 | 1.44789 | 47 | 2 | 1 | 7 | 1.44320 | 60 | 0 | 0 | 8 |
| 1.43016 | 6 | 3 | 1 | 6 | 1.39489 | 15 | 3 | 3 | 4 | 1.33904 | 80 | 4 | 2 | 4 |
| 1.33681 | 82 | 4 | 1 | 5 | 1.29207 | 25 | 5 | 1 | 2 | 1.26978 | 44 | 4 | 0 | 6 |
| 1.24791 | 22 | 5 | 2 | 1 | 1.23542 | 46 | 2 | 2 | 8 | 1.22722 | 61 | 3 | 3 | 6 |
| 1.19612 | 51 | 3 | 1 | 8 | 1.19497 | 16 | 4 | 4 | 0 | 1.19339 | 38 | 5 | 2 | 3 |
| 1.18866 | 13 | 4 | 2 | 6 | 1.18092 | 32 | 2 | 1 | 9 | 1.17017 | 11 | 4 | 4 | 2 |
| 1.16277 | 44 | 4 | 1 | 7 | 1.15929 | 33 | 5 | 3 | 0 | 1.13660 | 17 | 5 | 3 | 2 |
| 1.12663 | 51 | 6 | 0 | 0 | 1.12228 | 6 | 1 | 1 | 10 | 1.10602 | 10 | 6 | 1 | 1M |
| 1.10602 | 10 | 6 | 0 | 2M | 1.10409 | 15 | 4 | 4 | 4 | 1.10284 | 28 | 5 | 2 | 5 |
| 1.09232 | 62 | 2 | 0 | 10M | 1.09232 | 62 | 5 | 1 | 6M | 1.07577 | 60 | 5 | 3 | 4 |
| 1.06963 | 18 | 3 | 3 | 8 | 1.05104 | 104 | 5 | 4 | 1M | 1.05104 | 104 | 6 | 2 | 2M |
| 1.04952 | 69 | 6 | 0 | 4 | 1.04382 | 46 | 4 | 2 | 8 | 1.01809 | 30 | 5 | 4 | 3 |
| 1.01515 | 6 | 4 | 4 | 6 | 1.01032 | 34 | 4 | 1 | 9 | 0.99888 | 11 | 5 | 2 | 7 |
| 0.99301 | 11 | 5 | 3 | 6 | 0.99154 | 31 | 2 | 1 | 11 | 0.96213 | 15 | 0 | 0 | 12 |
| 0.96012 | 25 | 5 | 4 | 5 | 0.95332 | 18 | 4 | 0 | 10 | 0.94313 | 16 | 5 | 5 | 2M |
| 0.94313 | 16 | 7 | 1 | 2M | 0.93455 | 101 | 3 | 3 | 10M | 0.93455 | 101 | 6 | 2 | 6M |
| 0.92554 | 34 | 7 | 2 | 1 | 0.92529 | 44 | 6 | 4 | 2 | 0.92163 | 5 | 6 | 1 | 7 |
| 0.92041 | 17 | 4 | 4 | 8 | 0.91752 | 8 | 4 | 2 | 10 | 0.90381 | 48 | 5 | 3 | 8 |
| 0.90263 | 35 | 7 | 2 | 3 | 0.89720 | 13 | 5 | 2 | 9 | 0.89252 | 26 | 2 | 2 | 12 |
| 0.89157 | 10 | 6 | 4 | 4 | 0.88916 | 23 | 5 | 4 | 7 | 0.88802 | 84 | 6 | 0 | 8M |
| 0.88802 | 84 | 7 | 3 | 0M | 0.88397 | 40 | 4 | 1 | 11 | 0.87736 | 22 | 3 | 1 | 12 |
| 0.87066 | 14 | 5 | 1 | 10 | 0.86149 | 32 | 7 | 2 | 5 | 0.85615 | 17 | 7 | 1 | 6M |
| 0.85615 | 17 | 5 | 5 | 6M | 0.85211 | 27 | 2 | 1 | 13 | 0.84840 | 6 | 7 | 3 | 4 |
| 0.84273 | 47 | 6 | 4 | 6 | 0.83625 | 71 | 7 | 4 | 1M | 0.83625 | 71 | 8 | 1 | 1M |
| 0.83606 | 64 | 8 | 0 | 2+ | 0.82362 | 6 | 3 | 3 | 12 |
Table 11. X-ray diffraction pattern of BaSm2ZnO5 with d spacings (Å), Miller indices h k l, and integrated intensitiesI normalized to the value of 999 as the maximum.
| d | I | h | k | l | d | I | h | k | l | d | I | h | k | l |
| 6.29411 | 31 | 0 | 2 | 0 | 6.25287 | 49 | 1 | 1 | 0 | 4.74001 | 10 | 1 | 2 | 0 |
| 4.52364 | 66 | 1 | 0 | 1 | 3.67333 | 27 | 1 | 2 | 1 | 3.60226 | 19 | 2 | 0 | 0 |
| 3.46325 | 71 | 2 | 1 | 0 | 3.14705 | 129 | 0 | 4 | 0 | 3.12643 | 31 | 2 | 2 | 0 |
| 3.07636 | 999 | 1 | 3 | 1 | 2.97513 | 751 | 2 | 1 | 1 | 2.90609 | 592 | 0 | 0 | 2 |
| 2.88392 | 208 | 1 | 4 | 0 | 2.76742 | 105 | 0 | 4 | 1 | 2.75337 | 62 | 2 | 2 | 1 |
| 2.73323 | 21 | 2 | 3 | 0 | 2.58339 | 80 | 1 | 4 | 1 | 2.47363 | 157 | 2 | 3 | 1+ |
| 2.37636 | 98 | 1 | 5 | 0+ | 2.35896 | 24 | 3 | 1 | 0 | 2.26182 | 21 | 2 | 0 | 2 |
| 2.24373 | 114 | 3 | 2 | 0 | 2.22617 | 73 | 2 | 1 | 2 | 2.21951 | 54 | 3 | 0 | 1 |
| 2.19988 | 28 | 1 | 5 | 1 | 2.18579 | 57 | 3 | 1 | 1 | 2.13503 | 60 | 0 | 4 | 2 |
| 2.12855 | 17 | 2 | 2 | 2 | 2.09804 | 57 | 0 | 6 | 0 | 2.09318 | 16 | 3 | 2 | 1 |
| 2.04703 | 276 | 1 | 4 | 2 | 1.99099 | 115 | 2 | 3 | 2 | 1.97340 | 7 | 0 | 6 | 1 |
| 1.96195 | 23 | 3 | 3 | 1 | 1.94466 | 14 | 2 | 5 | 1 | 1.90914 | 142 | 3 | 4 | 0 |
| 1.90329 | 22 | 1 | 6 | 1 | 1.83978 | 51 | 1 | 5 | 2 | 1.81333 | 40 | 3 | 4 | 1M |
| 1.81333 | 40 | 2 | 6 | 0M | 1.80113 | 64 | 4 | 0 | 0 | 1.78297 | 14 | 4 | 1 | 0 |
| 1.77599 | 85 | 3 | 2 | 2 | 1.74478 | 49 | 1 | 7 | 0 | 1.73072 | 7 | 2 | 6 | 1 |
| 1.70877 | 145 | 1 | 3 | 3 | 1.70457 | 32 | 4 | 1 | 1 | 1.70106 | 27 | 0 | 6 | 2 |
| 1.69081 | 150 | 2 | 1 | 3 | 1.67111 | 46 | 1 | 7 | 1 | 1.66491 | 14 | 3 | 5 | 1 |
| 1.65554 | 11 | 1 | 6 | 2 | 1.64982 | 19 | 0 | 4 | 3 | 1.64683 | 12 | 2 | 2 | 3 |
| 1.60819 | 19 | 1 | 4 | 3 | 1.59562 | 137 | 3 | 4 | 2 | 1.58038 | 51 | 2 | 3 | 3M |
| 1.58038 | 51 | 3 | 6 | 0M | 1.57353 | 8 | 0 | 8 | 0 | 1.55065 | 104 | 2 | 7 | 1 |
| 1.53804 | 39 | 2 | 6 | 2M | 1.53804 | 39 | 1 | 8 | 0M | 1.53094 | 48 | 4 | 0 | 2 |
| 1.51919 | 47 | 4 | 1 | 2M | 1.51919 | 47 | 0 | 8 | 1M | 1.50923 | 75 | 4 | 4 | 1M |
| 1.50923 | 75 | 3 | 0 | 3M | 1.50168 | 7 | 1 | 5 | 3 | 1.49632 | 47 | 3 | 1 | 3M |
| 1.49632 | 47 | 1 | 7 | 2M | 1.48618 | 26 | 1 | 8 | 1 | 1.46554 | 16 | 3 | 2 | 3M |
| 1.46554 | 16 | 4 | 5 | 0M | 1.45305 | 94 | 0 | 0 | 4 | 1.43917 | 43 | 3 | 7 | 0M |
| 1.43917 | 43 | 4 | 3 | 2M | 1.42017 | 25 | 4 | 5 | 1M | 1.42017 | 25 | 3 | 3 | 3M |
| 1.40457 | 11 | 5 | 2 | 0 | 1.39857 | 10 | 5 | 0 | 1 | 1.39636 | 6 | 1 | 6 | 3 |
| 1.38811 | 24 | 3 | 6 | 2 | 1.38371 | 6 | 0 | 8 | 2 | 1.36661 | 13 | 4 | 6 | 0 |
| 1.35939 | 16 | 3 | 4 | 3M | 1.35939 | 16 | 1 | 8 | 2M | 1.33989 | 5 | 2 | 1 | 4 |
| 1.33627 | 8 | 1 | 9 | 1 | 1.33033 | 5 | 4 | 6 | 1 | 1.32681 | 90 | 5 | 3 | 1 |
| 1.31195 | 10 | 4 | 1 | 3 | 1.30808 | 6 | 4 | 5 | 2 | 1.29727 | 52 | 1 | 4 | 4M |
| 1.29727 | 52 | 1 | 7 | 3M | 1.28990 | 17 | 3 | 7 | 2 | 1.28366 | 43 | 3 | 8 | 1+ |
| 1.27223 | 6 | 2 | 9 | 1 | 1.26461 | 15 | 5 | 2 | 2 | 1.25882 | 8 | 0 | 10 | 0 |
| 1.25057 | 5 | 5 | 5 | 0 | 1.24315 | 28 | 4 | 7 | 1 | 1.23971 | 17 | 1 | 5 | 4 |
| 1.23778 | 57 | 2 | 7 | 3 | 1.23669 | 13 | 4 | 6 | 2 | 1.23030 | 14 | 0 | 10 | 1 |
| 1.22142 | 13 | 0 | 8 | 3 | 1.21963 | 28 | 3 | 2 | 4 | 1.21658 | 28 | 4 | 4 | 3 |
| 1.20424 | 16 | 1 | 8 | 3 | 1.20075 | 7 | 6 | 0 | 0 | 1.19893 | 5 | 3 | 8 | 2 |
| 1.19453 | 17 | 0 | 6 | 4 | 1.18835 | 5 | 2 | 10 | 0 | 1.18500 | 11 | 4 | 8 | 0 |
| 1.16846 | 13 | 4 | 5 | 3 | 1.16572 | 5 | 4 | 7 | 2 | 1.16371 | 7 | 5 | 6 | 1 |
| 1.16112 | 5 | 4 | 8 | 1 | 1.15624 | 48 | 3 | 4 | 4M | 1.15624 | 48 | 5 | 0 | 3M |
| 1.15475 | 21 | 0 | 10 | 2M | 1.15475 | 21 | 6 | 3 | 0 | 1.14873 | 6 | 5 | 5 | 2 |
| 1.14054 | 16 | 1 | 10 | 2 | 1.13382 | 10 | 2 | 6 | 4 | 1.13091 | 30 | 4 | 0 | 4 |
| 1.12637 | 6 | 4 | 1 | 4 | 1.12447 | 7 | 5 | 7 | 0 | 1.12025 | 5 | 1 | 9 | 3 |
| 1.11656 | 22 | 1 | 7 | 4 | 1.11469 | 57 | 3 | 10 | 0M | 1.11469 | 57 | 5 | 3 | 3M |
| 1.10964 | 24 | 6 | 0 | 2 | 1.10964 | 24 | 1 | 11 | 1M | 1.10694 | 41 | 1 | 3 | 5 |
| 1.10547 | 6 | 6 | 1 | 2 | 1.10400 | 7 | 5 | 7 | 1 | 1.10202 | 31 | 2 | 1 | 5 |
| 1.09994 | 8 | 2 | 10 | 2 | 1.09728 | 13 | 4 | 8 | 2 | 1.09062 | 36 | 2 | 11 | 0M |
| 1.09062 | 36 | 0 | 4 | 5M | 1.08870 | 17 | 3 | 8 | 3 | 1.08528 | 5 | 4 | 9 | 1 |
| 1.07815 | 7 | 1 | 4 | 5 | 1.07287 | 28 | 6 | 3 | 2 | 1.06962 | 21 | 2 | 3 | 5M |
| 1.06962 | 21 | 3 | 6 | 4M | 1.06751 | 6 | 0 | 8 | 4 | 1.06543 | 5 | 6 | 5 | 1 |
| 1.06365 | 15 | 4 | 7 | 3 | 1.05557 | 10 | 0 | 10 | 3 | 1.04870 | 16 | 5 | 7 | 2 |
| 1.04631 | 5 | 3 | 0 | 5 | 1.04232 | 16 | 3 | 1 | 5M | 1.04232 | 16 | 6 | 6 | 0M |
| 1.04095 | 19 | 3 | 10 | 2 | 1.03207 | 34 | 0 | 12 | 1+ | 1.02579 | 8 | 7 | 1 | 0M |
| 1.02579 | 8 | 6 | 6 | 1M | 1.02262 | 21 | 3 | 7 | 4 | 1.02112 | 56 | 2 | 11 | 2 |
| 1.01591 | 16 | 4 | 10 | 1 | 1.01345 | 18 | 7 | 0 | 1 | 1.01001 | 14 | 7 | 1 | 1M |
| 1.01001 | 14 | 5 | 2 | 4M | 0.99804 | 11 | 5 | 8 | 2 | 0.99550 | 12 | 4 | 6 | 4 |
| 0.98419 | 33 | 6 | 7 | 1 | 0.98098 | 28 | 6 | 6 | 2 | 0.97823 | 13 | 7 | 4 | 0 |
| 0.97624 | 7 | 1 | 11 | 3 | 0.97376 | 8 | 4 | 1 | 5 | 0.97240 | 15 | 5 | 7 | 3M |
| 0.97240 | 15 | 4 | 10 | 2 | 0.96870 | 19 | 0 | 0 | 6 | 0.96737 | 12 | 1 | 7 | 5M |
| 0.96737 | 12 | 7 | 1 | 2M | 0.95284 | 10 | 4 | 11 | 1 | 0.95143 | 9 | 0 | 10 | 4 |
| 0.94786 | 6 | 5 | 5 | 4 | 0.94687 | 17 | 1 | 13 | 1 | 0.94523 | 5 | 7 | 3 | 2 |
| 0.94225 | 29 | 2 | 7 | 5 | 0.93564 | 12 | 5 | 10 | 1 | 0.93498 | 9 | 0 | 8 | 5 |
| 0.93282 | 27 | 2 | 1 | 6M | 0.93282 | 27 | 4 | 4 | 5M | 0.92714 | 31 | 1 | 8 | 5 |
| 0.92714 | 31 | 7 | 4 | 2M | 0.92561 | 9 | 6 | 0 | 4 | 0.92325 | 5 | 2 | 13 | 1 |
| 0.92247 | 13 | 0 | 12 | 3 | 0.91989 | 6 | 2 | 10 | 4 | 0.91830 | 40 | 4 | 8 | 4M |
| 0.91830 | 40 | 1 | 4 | 6M | 0.91305 | 11 | 2 | 3 | 6 | 0.91070 | 13 | 4 | 10 | 3 |
Table 12. X-ray diffraction pattern of BaEu2ZnO5 with d spacings (Å), Miller indices h k l, and integrated intensitiesI normalized to the value of 999 as the maximum.
| d | I | h | k | l | d | I | h | k | l | d | I | h | k | l |
| 6.26795 | 33 | 0 | 2 | 0 | 6.22978 | 47 | 1 | 1 | 0 | 4.72157 | 15 | 1 | 2 | 0 |
| 4.50738 | 63 | 1 | 0 | 1 | 3.65943 | 11 | 1 | 2 | 1 | 3.58951 | 13 | 2 | 0 | 0 |
| 3.45083 | 54 | 2 | 1 | 0 | 3.13397 | 66 | 0 | 4 | 0 | 3.11489 | 19 | 2 | 2 | 0 |
| 3.06438 | 999 | 1 | 3 | 1 | 2.96443 | 815 | 2 | 1 | 1 | 2.89554 | 427 | 0 | 0 | 2 |
| 2.87222 | 194 | 1 | 4 | 0 | 2.75625 | 100 | 0 | 4 | 1 | 2.74324 | 67 | 2 | 2 | 1 |
| 2.72284 | 18 | 2 | 3 | 0 | 2.57312 | 92 | 1 | 4 | 1 | 2.46444 | 183 | 2 | 3 | 1+ |
| 2.36698 | 63 | 1 | 5 | 0 | 2.35056 | 15 | 3 | 1 | 0 | 2.25369 | 21 | 2 | 0 | 2 |
| 2.23562 | 125 | 3 | 2 | 0 | 2.21813 | 77 | 2 | 1 | 2 | 2.21163 | 56 | 3 | 0 | 1 |
| 2.19103 | 27 | 1 | 5 | 1 | 2.17799 | 62 | 3 | 1 | 1 | 2.12676 | 60 | 0 | 4 | 2 |
| 2.12077 | 13 | 2 | 2 | 2 | 2.08932 | 50 | 0 | 6 | 0 | 2.08560 | 14 | 3 | 2 | 1 |
| 2.03916 | 286 | 1 | 4 | 2 | 1.98358 | 92 | 2 | 3 | 2 | 1.95472 | 15 | 3 | 3 | 1 |
| 1.93704 | 5 | 2 | 5 | 1 | 1.90195 | 116 | 3 | 4 | 0 | 1.89557 | 18 | 1 | 6 | 1 |
| 1.83259 | 51 | 1 | 5 | 2 | 1.80624 | 36 | 3 | 4 | 1M | 1.80624 | 36 | 2 | 6 | 0M |
| 1.79476 | 66 | 4 | 0 | 0 | 1.77664 | 18 | 4 | 1 | 0 | 1.76956 | 113 | 3 | 2 | 2 |
| 1.73759 | 48 | 1 | 7 | 0 | 1.72385 | 7 | 2 | 6 | 1 | 1.70242 | 141 | 1 | 3 | 3 |
| 1.69851 | 32 | 4 | 1 | 1 | 1.69430 | 27 | 0 | 6 | 2 | 1.68469 | 138 | 2 | 1 | 3 |
| 1.66429 | 45 | 1 | 7 | 1 | 1.65855 | 12 | 3 | 5 | 1 | 1.64899 | 8 | 1 | 6 | 2 |
| 1.64360 | 17 | 0 | 4 | 3 | 1.64083 | 12 | 2 | 2 | 3 | 1.60214 | 21 | 1 | 4 | 3 |
| 1.58968 | 144 | 3 | 4 | 2 | 1.57444 | 54 | 2 | 3 | 3M | 1.57444 | 54 | 3 | 6 | 0M |
| 1.56699 | 8 | 0 | 8 | 0 | 1.54444 | 104 | 2 | 7 | 1 | 1.53196 | 48 | 2 | 6 | 2M |
| 1.53196 | 48 | 1 | 8 | 0M | 1.52548 | 52 | 4 | 0 | 2 | 1.51321 | 47 | 4 | 1 | 2M |
| 1.51321 | 47 | 0 | 8 | 1M | 1.50370 | 78 | 4 | 4 | 1M | 1.50370 | 78 | 3 | 0 | 3M |
| 1.49596 | 8 | 1 | 5 | 3 | 1.49178 | 17 | 3 | 1 | 3 | 1.48991 | 31 | 1 | 7 | 2 |
| 1.48010 | 30 | 1 | 8 | 1 | 1.45937 | 8 | 4 | 5 | 0 | 1.44777 | 82 | 0 | 0 | 4 |
| 1.43359 | 39 | 3 | 7 | 0M | 1.43359 | 39 | 4 | 3 | 2M | 1.41488 | 26 | 4 | 5 | 1M |
| 1.41488 | 26 | 3 | 3 | 3M | 1.40208 | 5 | 2 | 7 | 2 | 1.39955 | 12 | 5 | 2 | 0 |
| 1.39361 | 9 | 5 | 0 | 1 | 1.39097 | 5 | 1 | 6 | 3 | 1.38279 | 30 | 3 | 6 | 2 |
| 1.37812 | 7 | 0 | 8 | 2 | 1.36142 | 13 | 4 | 6 | 0 | 1.35425 | 10 | 3 | 4 | 3M |
| 1.35425 | 10 | 1 | 8 | 2M | 1.33504 | 7 | 2 | 1 | 4 | 1.33078 | 9 | 1 | 9 | 1 |
| 1.32203 | 88 | 5 | 3 | 1 | 1.31431 | 6 | 0 | 4 | 4 | 1.30725 | 14 | 4 | 1 | 3 |
| 1.30321 | 7 | 4 | 5 | 2 | 1.29282 | 30 | 1 | 4 | 4 | 1.29145 | 17 | 1 | 7 | 3 |
| 1.28490 | 20 | 3 | 7 | 2 | 1.27879 | 47 | 3 | 8 | 1+ | 1.26708 | 5 | 2 | 9 | 1 |
| 1.26008 | 13 | 5 | 2 | 2 | 1.25359 | 5 | 0 | 10 | 0 | 1.24596 | 6 | 5 | 5 | 0 |
| 1.23837 | 26 | 4 | 7 | 1 | 1.23644 | 5 | 1 | 9 | 2 | 1.23505 | 18 | 1 | 5 | 4+ |
| 1.23279 | 64 | 2 | 7 | 3+ | 1.22521 | 12 | 0 | 10 | 1 | 1.21660 | 15 | 0 | 8 | 3 |
| 1.21521 | 32 | 3 | 2 | 4 | 1.21212 | 30 | 4 | 4 | 3 | 1.20775 | 5 | 1 | 10 | 1 |
| 1.19950 | 15 | 1 | 8 | 3 | 1.19650 | 6 | 6 | 0 | 0 | 1.19424 | 5 | 3 | 8 | 2 |
| 1.18999 | 16 | 0 | 6 | 4 | 1.18349 | 7 | 2 | 10 | 0 | 1.18039 | 10 | 4 | 8 | 0 |
| 1.16413 | 14 | 4 | 5 | 3 | 1.15937 | 5 | 5 | 6 | 1 | 1.15661 | 5 | 4 | 8 | 1 |
| 1.15199 | 57 | 3 | 4 | 4+ | 1.15034 | 26 | 0 | 10 | 2M | 1.15034 | 26 | 6 | 3 | 0M |
| 1.14450 | 8 | 5 | 5 | 2 | 1.13591 | 15 | 1 | 10 | 2 | 1.12954 | 10 | 2 | 6 | 4 |
| 1.12685 | 32 | 4 | 0 | 4 | 1.12232 | 8 | 4 | 1 | 4 | 1.12022 | 6 | 5 | 7 | 0 |
| 1.11580 | 6 | 1 | 9 | 3 | 1.11228 | 23 | 1 | 7 | 4 | 1.11061 | 61 | 5 | 3 | 3+ |
| 1.10553 | 30 | 6 | 0 | 2M | 1.10553 | 30 | 1 | 11 | 1M | 1.10289 | 38 | 1 | 3 | 5 |
| 1.10154 | 8 | 6 | 1 | 2 | 1.09983 | 7 | 5 | 7 | 1 | 1.09802 | 34 | 2 | 1 | 5 |
| 1.09552 | 9 | 2 | 10 | 2 | 1.09306 | 14 | 4 | 8 | 2 | 1.08899 | 5 | 6 | 2 | 2 |
| 1.08618 | 37 | 2 | 11 | 0+ | 1.08449 | 20 | 3 | 8 | 3 | 1.08103 | 5 | 4 | 9 | 1 |
| 1.06901 | 26 | 6 | 3 | 2 | 1.06565 | 20 | 2 | 3 | 5M | 1.06565 | 20 | 3 | 6 | 4M |
| 1.06338 | 5 | 0 | 8 | 4 | 1.06154 | 5 | 6 | 5 | 1 | 1.05963 | 18 | 4 | 7 | 3 |
| 1.05135 | 8 | 0 | 10 | 3 | 1.04476 | 16 | 5 | 7 | 2 | 1.04265 | 11 | 6 | 4 | 2M |
| 1.04265 | 11 | 3 | 0 | 5M | 1.03851 | 18 | 3 | 1 | 5M | 1.03851 | 18 | 6 | 6 | 0M |
| 1.03682 | 17 | 3 | 10 | 2 | 1.02793 | 31 | 0 | 12 | 1+ | 1.02208 | 10 | 7 | 1 | 0M |
| 1.02208 | 10 | 6 | 6 | 1M | 1.01875 | 24 | 3 | 7 | 4 | 1.01700 | 62 | 2 | 11 | 2 |
| 1.01304 | 5 | 3 | 11 | 1 | 1.01191 | 14 | 4 | 10 | 1 | 1.00986 | 18 | 7 | 0 | 1 |
| 1.00641 | 18 | 7 | 1 | 1M | 1.00641 | 18 | 5 | 2 | 4M | 0.99425 | 10 | 5 | 8 | 2 |
| 0.99179 | 12 | 4 | 6 | 4 | 0.98052 | 31 | 6 | 7 | 1 | 0.97736 | 32 | 6 | 6 | 2 |
| 0.97471 | 14 | 7 | 4 | 0 | 0.97212 | 11 | 1 | 11 | 3M | 0.97212 | 11 | 3 | 8 | 4M |
| 0.97025 | 7 | 4 | 1 | 5 | 0.96864 | 18 | 5 | 7 | 3M | 0.96864 | 18 | 4 | 10 | 2M |
| 0.96518 | 25 | 0 | 0 | 6 | 0.96378 | 15 | 7 | 1 | 2M | 0.96378 | 15 | 1 | 7 | 5M |
| 0.94906 | 11 | 4 | 11 | 1 | 0.94769 | 8 | 0 | 10 | 4 | 0.94500 | 5 | 5 | 9 | 2 |
| 0.94438 | 6 | 5 | 5 | 4 | 0.94296 | 14 | 1 | 13 | 1 | 0.94185 | 6 | 7 | 3 | 2 |
| 0.93870 | 27 | 2 | 7 | 5 | 0.93176 | 20 | 5 | 10 | 1M | 0.93176 | 20 | 0 | 8 | 5M |
| 0.92942 | 22 | 2 | 1 | 6M | 0.92942 | 22 | 4 | 4 | 5M | 0.92374 | 31 | 7 | 4 | 2M |
| 0.92374 | 31 | 1 | 8 | 5M | 0.92235 | 14 | 0 | 4 | 6M | 0.92235 | 14 | 6 | 0 | 4M |
| 0.91875 | 14 | 0 | 12 | 3 | 0.91630 | 7 | 2 | 10 | 4 | 0.91490 | 38 | 1 | 4 | 6M |
| 0.91490 | 38 | 4 | 8 | 4M | 0.90972 | 9 | 2 | 3 | 6 | 0.90798 | 5 | 3 | 11 | 3 |
Table 13. X-ray diffraction pattern of BaGd2ZnO5 with d spacings (Å), Miller indices h k l, and integrated intensitiesI normalized to the value of 999 as the maximum.
| d | I | h | k | l | d | I | h | k | l | d | I | h | k | l |
| 6.24700 | 30 | 0 | 2 | 0 | 6.21045 | 51 | 1 | 1 | 0 | 4.49408 | 62 | 1 | 0 | 1 |
| 3.64814 | 10 | 1 | 2 | 1 | 3.57865 | 15 | 2 | 0 | 0 | 3.44031 | 62 | 2 | 1 | 0 |
| 3.12350 | 85 | 0 | 4 | 0 | 3.10523 | 31 | 2 | 2 | 0 | 3.05469 | 999* | 1 | 3 | 1 |
| 2.95550 | 847 | 2 | 1 | 1 | 2.88713 | 525 | 0 | 0 | 2 | 2.86276 | 217 | 1 | 4 | 0 |
| 2.74731 | 112 | 0 | 4 | 1 | 2.73485 | 74 | 2 | 2 | 1 | 2.71424 | 22 | 2 | 3 | 0 |
| 2.56485 | 93 | 1 | 4 | 1 | 2.45665 | 156 | 2 | 3 | 1+ | 2.35915 | 77 | 1 | 5 | 0 |
| 2.35322 | 5 | 2 | 4 | 0 | 2.34343 | 19 | 3 | 1 | 0 | 2.24704 | 27 | 2 | 0 | 2 |
| 2.22876 | 132 | 3 | 2 | 0 | 2.21156 | 84 | 2 | 1 | 2 | 2.20497 | 56 | 3 | 0 | 1 |
| 2.18391 | 30 | 1 | 5 | 1 | 2.17920 | 6 | 2 | 4 | 1 | 2.17142 | 69 | 3 | 1 | 1 |
| 2.12015 | 66 | 0 | 4 | 2 | 2.11441 | 18 | 2 | 2 | 2 | 2.08173 | 77 | 0 | 6 | 0M |
| 2.08173 | 77 | 3 | 2 | 1M | 2.03284 | 318 | 1 | 4 | 2 | 1.99943 | 5 | 1 | 6 | 0 |
| 1.97755 | 115 | 2 | 3 | 2 | 1.95885 | 5 | 0 | 6 | 1 | 1.94870 | 17 | 3 | 3 | 1 |
| 1.93084 | 7 | 2 | 5 | 1 | 1.89597 | 126 | 3 | 4 | 0 | 1.88937 | 20 | 1 | 6 | 1 |
| 1.85872 | 7 | 1 | 0 | 3 | 1.82683 | 54 | 1 | 5 | 2 | 1.80051 | 42 | 3 | 4 | 1M |
| 1.80051 | 42 | 2 | 6 | 0M | 1.78933 | 72 | 4 | 0 | 0 | 1.77125 | 18 | 4 | 1 | 0 |
| 1.76424 | 113 | 3 | 2 | 2 | 1.73182 | 58 | 1 | 7 | 0 | 1.71828 | 12 | 2 | 6 | 1 |
| 1.69734 | 157 | 1 | 3 | 3 | 1.69338 | 36 | 4 | 1 | 1 | 1.68889 | 26 | 0 | 6 | 2 |
| 1.67974 | 147 | 2 | 1 | 3 | 1.65882 | 47 | 1 | 7 | 1 | 1.65332 | 12 | 3 | 5 | 1 |
| 1.64374 | 9 | 1 | 6 | 2 | 1.63862 | 21 | 0 | 4 | 3 | 1.63597 | 14 | 2 | 2 | 3 |
| 1.59730 | 22 | 1 | 4 | 3+ | 1.58480 | 153 | 3 | 4 | 2 | 1.56961 | 54 | 2 | 3 | 3M |
| 1.56961 | 54 | 3 | 6 | 0M | 1.56175 | 10 | 0 | 8 | 0 | 1.53941 | 102 | 2 | 7 | 1 |
| 1.52708 | 46 | 2 | 6 | 2M | 1.52708 | 46 | 1 | 8 | 0M | 1.52092 | 53 | 4 | 0 | 2 |
| 1.50977 | 18 | 4 | 1 | 2 | 1.50758 | 35 | 0 | 8 | 1 | 1.49911 | 79 | 4 | 4 | 1M |
| 1.49911 | 79 | 3 | 0 | 3M | 1.49137 | 8 | 1 | 5 | 3 | 1.48737 | 21 | 3 | 1 | 3 |
| 1.48513 | 34 | 1 | 7 | 2 | 1.47521 | 35 | 1 | 8 | 1 | 1.45480 | 11 | 4 | 5 | 0 |
| 1.44357 | 101 | 0 | 0 | 4 | 1.42903 | 46 | 3 | 7 | 0M | 1.42903 | 46 | 4 | 3 | 2M |
| 1.41054 | 31 | 4 | 5 | 1M | 1.41054 | 31 | 3 | 3 | 3M | 1.39761 | 7 | 2 | 7 | 2 |
| 1.39530 | 13 | 5 | 2 | 0 | 1.38940 | 11 | 5 | 0 | 1 | 1.38666 | 5 | 1 | 6 | 3 |
| 1.37845 | 34 | 3 | 6 | 2 | 1.37365 | 7 | 0 | 8 | 2 | 1.35712 | 15 | 4 | 6 | 0+ |
| 1.35072 | 8 | 3 | 4 | 3 | 1.33113 | 8 | 2 | 1 | 4 | 1.32638 | 9 | 1 | 9 | 1 |
| 1.31799 | 95 | 5 | 3 | 1 | 1.31039 | 6 | 0 | 4 | 4 | 1.30336 | 12 | 4 | 1 | 3 |
| 1.29919 | 9 | 4 | 5 | 2 | 1.29425 | 6 | 2 | 9 | 0 | 1.28896 | 35 | 1 | 4 | 4 |
| 1.28740 | 20 | 1 | 7 | 3 | 1.28083 | 18 | 3 | 7 | 2 | 1.27578 | 10 | 5 | 1 | 2 |
| 1.27446 | 36 | 2 | 3 | 4M | 1.27446 | 36 | 3 | 8 | 1M | 1.26322 | 10 | 4 | 7 | 0M |
| 1.26322 | 10 | 2 | 9 | 1M | 1.25628 | 16 | 5 | 2 | 2 | 1.24940 | 8 | 0 | 10 | 0 |
| 1.24209 | 9 | 5 | 5 | 0 | 1.23445 | 30 | 4 | 7 | 1 | 1.23134 | 19 | 1 | 5 | 4+ |
| 1.22913 | 50 | 2 | 7 | 3+ | 1.22820 | 13 | 4 | 6 | 2 | 1.22114 | 14 | 0 | 10 | 1 |
| 1.21275 | 16 | 0 | 8 | 3 | 1.21162 | 34 | 3 | 2 | 4 | 1.20845 | 32 | 4 | 4 | 3 |
| 1.20375 | 5 | 1 | 10 | 1 | 1.19570 | 17 | 1 | 8 | 3 | 1.19288 | 6 | 6 | 0 | 0 |
| 1.19043 | 5 | 3 | 8 | 2 | 1.18637 | 17 | 0 | 6 | 4 | 1.17958 | 7 | 2 | 10 | 0 |
| 1.17661 | 9 | 4 | 8 | 0 | 1.16058 | 18 | 4 | 5 | 3 | 1.15763 | 5 | 4 | 7 | 2 |
| 1.14863 | 7 | 5 | 0 | 3 | 1.14855 | 50 | 3 | 4 | 4+ | 1.14670 | 25 | 6 | 3 | 0M |
| 1.14670 | 25 | 0 | 10 | 2M | 1.14098 | 8 | 5 | 5 | 2 | 1.13220 | 17 | 1 | 10 | 2 |
| 1.12610 | 12 | 2 | 6 | 4 | 1.12352 | 31 | 4 | 0 | 4 | 1.11900 | 8 | 4 | 1 | 4 |
| 1.11669 | 8 | 5 | 7 | 0 | 1.11225 | 6 | 1 | 9 | 3 | 1.10886 | 25 | 1 | 7 | 4+ |
| 1.10729 | 58 | 5 | 3 | 3 | 1.10681 | 8 | 3 | 10 | 0 | 1.10249 | 20 | 6 | 0 | 2 |
| 1.10119 | 7 | 1 | 11 | 1 | 1.09965 | 45 | 1 | 3 | 5 | 1.09822 | 8 | 6 | 1 | 2 |
| 1.09638 | 7 | 5 | 7 | 1 | 1.09482 | 37 | 2 | 1 | 5+ | 1.09196 | 9 | 2 | 10 | 2 |
| 1.08960 | 13 | 4 | 8 | 2 | 1.08260 | 38 | 2 | 11 | 0+ | 1.08109 | 20 | 3 | 8 | 3 |
| 1.07756 | 6 | 4 | 9 | 1 | 1.07099 | 8 | 1 | 4 | 5 | 1.06578 | 27 | 6 | 3 | 2 |
| 1.06247 | 24 | 2 | 3 | 5M | 1.06247 | 24 | 3 | 6 | 4M | 1.05828 | 6 | 6 | 5 | 1 |
| 1.05635 | 17 | 4 | 7 | 3 | 1.04797 | 11 | 0 | 10 | 3 | 1.04150 | 14 | 5 | 7 | 2 |
| 1.03508 | 13 | 6 | 6 | 0 | 1.03347 | 19 | 3 | 10 | 2 | 1.02464 | 34 | 0 | 12 | 1+ |
| 1.01893 | 10 | 7 | 1 | 0M | 1.01893 | 10 | 6 | 6 | 1M | 1.01563 | 21 | 3 | 7 | 4 |
| 1.01368 | 67 | 2 | 11 | 2 | 1.00973 | 5 | 3 | 11 | 1 | 1.00864 | 15 | 4 | 10 | 1+ |
| 1.00681 | 20 | 7 | 0 | 1 | 1.00340 | 22 | 7 | 1 | 1M | 1.00340 | 22 | 5 | 2 | 4M |
| 0.99298 | 5 | 7 | 3 | 0 | 0.99113 | 12 | 5 | 8 | 2 | 0.98878 | 11 | 4 | 6 | 4 |
| 0.97746 | 30 | 6 | 7 | 1 | 0.97435 | 31 | 6 | 6 | 2 | 0.97173 | 13 | 7 | 4 | 0 |
| 0.96899 | 11 | 1 | 11 | 3M | 0.96899 | 11 | 3 | 8 | 4M | 0.96740 | 10 | 4 | 1 | 5 |
| 0.96556 | 20 | 5 | 7 | 3M | 0.96556 | 20 | 4 | 10 | 2M | 0.96238 | 22 | 0 | 0 | 6 |
| 0.96087 | 15 | 7 | 1 | 2M | 0.96087 | 15 | 1 | 7 | 5M | 0.95276 | 12 | 4 | 9 | 3M |
| 0.95276 | 12 | 7 | 2 | 2M | 0.95083 | 6 | 4 | 7 | 4 | 0.94598 | 12 | 4 | 11 | 1 |
| 0.94471 | 9 | 0 | 10 | 4 | 0.94178 | 16 | 5 | 9 | 2M | 0.94178 | 16 | 5 | 5 | 4M |
| 0.93976 | 20 | 1 | 13 | 1M | 0.93976 | 20 | 6 | 5 | 3M | 0.93900 | 7 | 7 | 3 | 2 |
| 0.93585 | 32 | 2 | 7 | 5 | 0.92884 | 25 | 5 | 10 | 1M | 0.92884 | 25 | 0 | 8 | 5M |
| 0.92668 | 26 | 2 | 1 | 6M | 0.92668 | 26 | 4 | 4 | 5M | 0.92096 | 27 | 7 | 4 | 2+ |
Table 14. X-ray diffraction pattern of BaDy2ZnO5 with d spacings (Å), Miller indices h k l, and integrated intensities I normalized to the value of 999 as the maximum.
| d | I | h | k | l | d | I | h | k | l | d | I | h | k | l |
| 6.19234 | 25 | 0 | 2 | 0 | 6.15523 | 32 | 1 | 1 | 0 | 4.66487 | 7 | 1 | 2 | 0 |
| 4.45727 | 66 | 1 | 0 | 1 | 3.61756 | 13 | 1 | 2 | 1 | 3.54666 | 12 | 2 | 0 | 0 |
| 3.40961 | 58 | 2 | 1 | 0 | 3.09617 | 63 | 0 | 4 | 0 | 3.07761 | 24 | 2 | 2 | 0 |
| 3.02875 | 999 | 1 | 3 | 1 | 2.93007 | 827 | 2 | 1 | 1 | 2.86490 | 515 | 0 | 0 | 2 |
| 2.83763 | 233 | 1 | 4 | 0 | 2.72392 | 112 | 0 | 4 | 1 | 2.71126 | 71 | 2 | 2 | 1 |
| 2.69019 | 18 | 2 | 3 | 0 | 2.54287 | 105 | 1 | 4 | 1 | 2.44127 | 12 | 1 | 2 | 2 |
| 2.43515 | 160 | 2 | 3 | 1 | 2.33846 | 89 | 1 | 5 | 0 | 2.33244 | 6 | 2 | 4 | 0 |
| 2.32250 | 18 | 3 | 1 | 0 | 2.22863 | 29 | 2 | 0 | 2 | 2.20889 | 138 | 3 | 2 | 0 |
| 2.19340 | 96 | 2 | 1 | 2 | 2.18566 | 61 | 3 | 0 | 1 | 2.16031 | 7 | 2 | 4 | 1 |
| 2.15240 | 73 | 3 | 1 | 1 | 2.10280 | 60 | 0 | 4 | 2 | 2.09696 | 17 | 2 | 2 | 2 |
| 2.06345 | 75 | 0 | 6 | 0M | 2.06345 | 75 | 3 | 2 | 1M | 2.01608 | 315 | 1 | 4 | 2 |
| 1.98191 | 7 | 1 | 6 | 0 | 1.96111 | 118 | 2 | 3 | 2 | 1.94195 | 5 | 0 | 6 | 1 |
| 1.93164 | 18 | 3 | 3 | 1 | 1.91407 | 9 | 2 | 5 | 1 | 1.87916 | 134 | 3 | 4 | 0 |
| 1.87302 | 21 | 1 | 6 | 1 | 1.81159 | 53 | 1 | 5 | 2 | 1.78468 | 39 | 3 | 4 | 1M |
| 1.78468 | 39 | 2 | 6 | 0M | 1.77333 | 60 | 4 | 0 | 0 | 1.75543 | 20 | 4 | 1 | 0 |
| 1.74931 | 114 | 3 | 2 | 2 | 1.71665 | 61 | 1 | 7 | 0 | 1.70333 | 12 | 2 | 6 | 1 |
| 1.68386 | 192 | 1 | 3 | 3 | 1.67842 | 42 | 4 | 1 | 1 | 1.67471 | 26 | 0 | 6 | 2 |
| 1.66631 | 171 | 2 | 1 | 3 | 1.64443 | 48 | 1 | 7 | 1 | 1.63885 | 11 | 3 | 5 | 1 |
| 1.62990 | 10 | 1 | 6 | 2 | 1.62553 | 19 | 0 | 4 | 3 | 1.62283 | 17 | 2 | 2 | 3 |
| 1.58446 | 22 | 1 | 4 | 3+ | 1.57130 | 138 | 3 | 4 | 2 | 1.55735 | 41 | 2 | 3 | 3 |
| 1.55496 | 23 | 3 | 6 | 0 | 1.54808 | 7 | 0 | 8 | 0 | 1.52600 | 99 | 2 | 7 | 1 |
| 1.51406 | 49 | 2 | 6 | 2M | 1.51406 | 49 | 1 | 8 | 0M | 1.50784 | 51 | 4 | 0 | 2 |
| 1.49679 | 17 | 4 | 1 | 2 | 1.49450 | 30 | 0 | 8 | 1 | 1.48607 | 73 | 4 | 4 | 1M |
| 1.48607 | 73 | 3 | 0 | 3M | 1.47925 | 8 | 1 | 5 | 3 | 1.47518 | 21 | 3 | 1 | 3 |
| 1.47253 | 35 | 1 | 7 | 2 | 1.46239 | 32 | 1 | 8 | 1 | 1.44189 | 10 | 4 | 5 | 0 |
| 1.43245 | 75 | 0 | 0 | 4 | 1.41650 | 44 | 3 | 7 | 0M | 1.41650 | 44 | 4 | 3 | 2M |
| 1.39830 | 26 | 4 | 5 | 1+ | 1.38568 | 7 | 2 | 7 | 2 | 1.38284 | 12 | 5 | 2 | 0 |
| 1.37708 | 10 | 5 | 0 | 1 | 1.37527 | 6 | 1 | 6 | 3+ | 1.36663 | 28 | 3 | 6 | 2 |
| 1.36196 | 5 | 0 | 8 | 2 | 1.34510 | 11 | 4 | 6 | 0 | 1.33951 | 5 | 3 | 4 | 3 |
| 1.33753 | 5 | 1 | 8 | 2 | 1.32063 | 6 | 2 | 1 | 4 | 1.31484 | 12 | 1 | 9 | 1 |
| 1.30632 | 86 | 5 | 3 | 1 | 1.30372 | 5 | 2 | 6 | 3 | 1.30005 | 7 | 0 | 4 | 4 |
| 1.29517 | 5 | 3 | 8 | 0 | 1.29245 | 13 | 4 | 1 | 3 | 1.28796 | 8 | 4 | 5 | 2 |
| 1.27875 | 34 | 1 | 4 | 4 | 1.27673 | 20 | 1 | 7 | 3 | 1.26982 | 19 | 3 | 7 | 2 |
| 1.26459 | 13 | 5 | 1 | 2M | 1.26459 | 13 | 2 | 3 | 4M | 1.26330 | 34 | 3 | 8 | 1 |
| 1.25216 | 9 | 4 | 7 | 0M | 1.25216 | 9 | 2 | 9 | 1M | 1.24535 | 16 | 5 | 2 | 2 |
| 1.23847 | 8 | 0 | 10 | 0 | 1.23105 | 6 | 5 | 5 | 0 | 1.22359 | 25 | 4 | 7 | 1 |
| 1.22157 | 23 | 1 | 9 | 2M | 1.22157 | 23 | 1 | 5 | 4M | 1.21888 | 42 | 2 | 7 | 3 |
| 1.21757 | 11 | 4 | 6 | 2 | 1.21051 | 11 | 0 | 10 | 1 | 1.20211 | 47 | 0 | 8 | 3M |
| 1.20211 | 47 | 3 | 2 | 4M | 1.19827 | 22 | 4 | 4 | 3 | 1.18572 | 15 | 1 | 8 | 3 |
| 1.18222 | 7 | 6 | 0 | 0 | 1.18017 | 6 | 3 | 8 | 2 | 1.17683 | 17 | 0 | 6 | 4+ |
| 1.16923 | 6 | 2 | 10 | 0 | 1.16622 | 5 | 4 | 8 | 0 | 1.15078 | 15 | 4 | 5 | 3 |
| 1.14761 | 6 | 4 | 7 | 2 | 1.14554 | 5 | 5 | 6 | 1 | 1.14279 | 5 | 4 | 8 | 1 |
| 1.13921 | 44 | 3 | 4 | 4 | 1.13858 | 13 | 5 | 0 | 3M | 1.13858 | 13 | 6 | 2 | 1M |
| 1.13669 | 23 | 0 | 10 | 2M | 1.13669 | 23 | 6 | 3 | 0M | 1.13105 | 8 | 5 | 5 | 2 |
| 1.12247 | 16 | 1 | 10 | 2 | 1.11694 | 10 | 2 | 6 | 4 | 1.11432 | 27 | 4 | 0 | 4 |
| 1.10983 | 9 | 4 | 1 | 4 | 1.10445 | 5 | 6 | 4 | 0 | 1.10290 | 6 | 1 | 9 | 3 |
| 1.09984 | 23 | 1 | 7 | 4+ | 1.09777 | 59 | 5 | 3 | 3M | 1.09777 | 59 | 3 | 10 | 0M |
| 1.09283 | 21 | 6 | 0 | 2 | 1.09106 | 45 | 1 | 3 | 5 | 1.08860 | 8 | 6 | 1 | 2 |
| 1.08634 | 43 | 5 | 7 | 1M | 1.08634 | 43 | 2 | 1 | 5M | 1.08255 | 11 | 2 | 10 | 2 |
| 1.08015 | 10 | 4 | 8 | 2 | 1.07609 | 7 | 6 | 2 | 2+ | 1.07311 | 30 | 2 | 11 | 0 |
| 1.07195 | 20 | 3 | 8 | 3 | 1.06810 | 7 | 4 | 9 | 1 | 1.06258 | 7 | 1 | 4 | 5+ |
| 1.05644 | 16 | 6 | 3 | 2 | 1.05387 | 21 | 2 | 3 | 5M | 1.05387 | 21 | 3 | 6 | 4M |
| 1.04890 | 6 | 6 | 5 | 1 | 1.04737 | 17 | 4 | 7 | 3 | 1.04591 | 5 | 5 | 8 | 0 |
| 1.03950 | 17 | 1 | 8 | 4M | 1.03950 | 17 | 0 | 10 | 3M | 1.03243 | 10 | 5 | 7 | 2 |
| 1.03052 | 6 | 6 | 4 | 2 | 1.02767 | 6 | 3 | 1 | 5 | 1.02587 | 13 | 6 | 6 | 0 |
| 1.02453 | 14 | 3 | 10 | 2 | 1.01576 | 28 | 0 | 12 | 1+ | 1.00988 | 13 | 7 | 1 | 0M |
| 1.00988 | 13 | 6 | 6 | 1M | 1.00723 | 21 | 3 | 7 | 4 | 1.00492 | 69 | 2 | 11 | 2 |
| 0.99979 | 14 | 4 | 10 | 1 | 0.99785 | 13 | 7 | 0 | 1+ | 0.99479 | 19 | 5 | 2 | 4M |
| 0.99479 | 19 | 7 | 1 | 1M | 0.98249 | 10 | 5 | 8 | 2 | 0.98055 | 11 | 4 | 6 | 4 |
| 0.96881 | 26 | 6 | 7 | 1 | 0.96582 | 30 | 6 | 6 | 2 | 0.96306 | 10 | 7 | 4 | 0 |
| 0.96070 | 6 | 3 | 8 | 4 | 0.95959 | 7 | 4 | 1 | 5 | 0.95723 | 18 | 5 | 7 | 3M |
| 0.95723 | 18 | 4 | 10 | 2M | 0.95511 | 24 | 2 | 9 | 4M | 0.95511 | 24 | 0 | 0 | 6M |
| 0.95291 | 16 | 1 | 7 | 5M | 0.95291 | 16 | 7 | 1 | 2M | 0.94481 | 5 | 4 | 9 | 3 |
| 0.94416 | 5 | 7 | 2 | 2 | 0.94289 | 5 | 4 | 7 | 4 | 0.93768 | 12 | 4 | 11 | 1 |
| 0.93686 | 7 | 0 | 10 | 4 | 0.93373 | 13 | 5 | 9 | 2M | 0.93373 | 13 | 5 | 5 | 4M |
| 0.93163 | 14 | 1 | 13 | 1+ | 0.93074 | 5 | 7 | 3 | 2 | 0.92830 | 22 | 2 | 7 | 5 |
Table 15. X-ray diffraction pattern of BaHo2ZnO5 with d spacings (Å), Miller indices h k l, and integrated intensities I normalized to the value of 999 as the maximum.
| d | I | h | k | l | d | I | h | k | l | d | I | h | k | l |
| 6.17101 | 15 | 0 | 2 | 0 | 6.13550 | 17 | 1 | 1 | 0 | 4.44320 | 29 | 1 | 0 | 1 |
| 3.60579 | 11 | 1 | 2 | 1 | 3.53558 | 16 | 2 | 0 | 0 | 3.39886 | 65 | 2 | 1 | 0 |
| 3.08551 | 83 | 0 | 4 | 0 | 3.06775 | 22 | 2 | 2 | 0 | 3.01872 | 999 | 1 | 3 | 1 |
| 2.92082 | 820 | 2 | 1 | 1 | 2.85580 | 489 | 0 | 0 | 2 | 2.82800 | 222 | 1 | 4 | 0 |
| 2.71471 | 90 | 0 | 4 | 1 | 2.70259 | 79 | 2 | 2 | 1 | 2.68142 | 8 | 2 | 3 | 0 |
| 2.58908 | 9 | 1 | 1 | 2 | 2.53436 | 127 | 1 | 4 | 1 | 2.43343 | 9 | 1 | 2 | 2 |
| 2.42724 | 164 | 2 | 3 | 1 | 2.33049 | 99 | 1 | 5 | 0 | 2.32472 | 6 | 2 | 4 | 0 |
| 2.31521 | 18 | 3 | 1 | 0 | 2.22160 | 32 | 2 | 0 | 2 | 2.20190 | 156 | 3 | 2 | 0 |
| 2.18646 | 88 | 2 | 1 | 2 | 2.17881 | 53 | 3 | 0 | 1 | 2.15778 | 30 | 1 | 5 | 1 |
| 2.15320 | 7 | 2 | 4 | 1 | 2.14563 | 86 | 3 | 1 | 1 | 2.09586 | 87 | 0 | 4 | 2 |
| 2.09027 | 22 | 2 | 2 | 2 | 2.05654 | 87 | 0 | 6 | 0M | 2.05654 | 87 | 3 | 2 | 1M |
| 2.00945 | 301 | 1 | 4 | 2 | 1.97513 | 7 | 1 | 6 | 0 | 1.95479 | 96 | 2 | 3 | 2 |
| 1.93532 | 5 | 0 | 6 | 1 | 1.92545 | 13 | 3 | 3 | 1 | 1.90771 | 13 | 2 | 5 | 1 |
| 1.87306 | 113 | 3 | 4 | 0 | 1.86667 | 20 | 1 | 6 | 1 | 1.80558 | 69 | 1 | 5 | 2 |
| 1.77879 | 47 | 3 | 4 | 1M | 1.77879 | 47 | 2 | 6 | 0M | 1.76779 | 66 | 4 | 0 | 0 |
| 1.74993 | 26 | 4 | 1 | 0 | 1.74376 | 141 | 3 | 2 | 2 | 1.71077 | 57 | 1 | 7 | 0 |
| 1.69763 | 14 | 2 | 6 | 1 | 1.67844 | 163 | 1 | 3 | 3 | 1.67316 | 36 | 4 | 1 | 1 |
| 1.66910 | 24 | 0 | 6 | 2 | 1.66103 | 143 | 2 | 1 | 3 | 1.63883 | 54 | 1 | 7 | 1 |
| 1.63349 | 13 | 3 | 5 | 1 | 1.62446 | 11 | 1 | 6 | 2 | 1.62025 | 15 | 0 | 4 | 3 |
| 1.61766 | 14 | 2 | 2 | 3 | 1.57932 | 26 | 1 | 4 | 3 | 1.56623 | 132 | 3 | 4 | 2 |
| 1.55236 | 37 | 2 | 3 | 3 | 1.54982 | 21 | 3 | 6 | 0 | 1.54275 | 7 | 0 | 8 | 0 |
| 1.52087 | 87 | 2 | 7 | 1 | 1.50904 | 51 | 2 | 6 | 2M | 1.50904 | 51 | 1 | 8 | 0M |
| 1.50311 | 44 | 4 | 0 | 2 | 1.49208 | 22 | 4 | 1 | 2 | 1.48938 | 36 | 0 | 8 | 1 |
| 1.48132 | 60 | 4 | 4 | 1M | 1.48132 | 60 | 3 | 0 | 3M | 1.47441 | 7 | 1 | 5 | 3 |
| 1.47052 | 29 | 3 | 1 | 3 | 1.46759 | 32 | 1 | 7 | 2 | 1.45740 | 29 | 1 | 8 | 1 |
| 1.43723 | 12 | 4 | 5 | 0 | 1.42790 | 88 | 0 | 0 | 4 | 1.41184 | 49 | 3 | 7 | 0M |
| 1.41184 | 49 | 4 | 3 | 2 | 1.39378 | 30 | 4 | 5 | 1+ | 1.38674 | 5 | 2 | 5 | 3 |
| 1.38106 | 11 | 2 | 7 | 2 | 1.37849 | 15 | 5 | 2 | 0 | 1.37277 | 11 | 5 | 0 | 1 |
| 1.37074 | 6 | 1 | 6 | 3+ | 1.36216 | 36 | 3 | 6 | 2 | 1.34071 | 14 | 4 | 6 | 0 |
| 1.33521 | 8 | 3 | 4 | 3 | 1.33302 | 6 | 1 | 8 | 2 | 1.31645 | 6 | 2 | 1 | 4 |
| 1.31035 | 10 | 1 | 9 | 1 | 1.30219 | 85 | 5 | 3 | 1 | 1.29945 | 5 | 2 | 6 | 3 |
| 1.29586 | 7 | 0 | 4 | 4 | 1.29083 | 5 | 3 | 8 | 0 | 1.28838 | 14 | 4 | 1 | 3 |
| 1.28381 | 9 | 4 | 5 | 2 | 1.27464 | 30 | 1 | 4 | 4 | 1.27250 | 19 | 1 | 7 | 3 |
| 1.26563 | 15 | 3 | 7 | 2 | 1.26060 | 10 | 5 | 1 | 2M | 1.26060 | 10 | 2 | 3 | 4M |
| 1.25908 | 24 | 3 | 8 | 1 | 1.24796 | 12 | 4 | 7 | 0M | 1.24796 | 12 | 2 | 9 | 1M |
| 1.24143 | 14 | 5 | 2 | 2 | 1.23420 | 8 | 0 | 10 | 0 | 1.22710 | 6 | 5 | 5 | 0 |
| 1.21958 | 24 | 4 | 7 | 1 | 1.21759 | 24 | 1 | 9 | 2M | 1.21759 | 24 | 1 | 5 | 4M |
| 1.21460 | 47 | 2 | 7 | 3M | 1.21460 | 47 | 4 | 6 | 2M | 1.20636 | 11 | 0 | 10 | 1 |
| 1.19822 | 49 | 0 | 8 | 3M | 1.19822 | 49 | 3 | 2 | 4M | 1.19445 | 23 | 4 | 4 | 3 |
| 1.18177 | 17 | 1 | 8 | 3 | 1.17853 | 6 | 6 | 0 | 0 | 1.17626 | 5 | 3 | 8 | 2 |
| 1.17299 | 15 | 0 | 6 | 4 | 1.16525 | 7 | 2 | 10 | 0 | 1.16236 | 7 | 4 | 8 | 0 |
| 1.15739 | 6 | 6 | 2 | 0M | 1.15739 | 6 | 1 | 6 | 4M | 1.14708 | 16 | 4 | 5 | 3 |
| 1.14386 | 6 | 4 | 7 | 2 | 1.13552 | 46 | 3 | 4 | 4M | 1.13552 | 46 | 5 | 0 | 3M |
| 1.13294 | 20 | 6 | 3 | 0M | 1.13294 | 20 | 0 | 10 | 2M | 1.12743 | 7 | 5 | 5 | 2 |
| 1.11866 | 13 | 1 | 10 | 2 | 1.11332 | 10 | 2 | 6 | 4 | 1.11080 | 26 | 4 | 0 | 4 |
| 1.10633 | 7 | 4 | 1 | 4 | 1.09921 | 5 | 1 | 9 | 3 | 1.09623 | 19 | 1 | 7 | 4+ |
| 1.09336 | 6 | 3 | 10 | 0+ | 1.08941 | 17 | 6 | 0 | 2 | 1.08758 | 35 | 1 | 3 | 5 |
| 1.08519 | 8 | 6 | 1 | 2 | 1.08286 | 36 | 5 | 7 | 1M | 1.08286 | 36 | 2 | 1 | 5M |
| 1.07889 | 9 | 2 | 10 | 2 | 1.07669 | 9 | 4 | 8 | 2 | 1.07282 | 5 | 6 | 2 | 2 |
| 1.06944 | 26 | 2 | 11 | 0 | 1.06841 | 16 | 3 | 8 | 3 | 1.06455 | 6 | 4 | 9 | 1 |
| 1.06141 | 5 | 2 | 9 | 3 | 1.05908 | 9 | 1 | 4 | 5+ | 1.05311 | 19 | 6 | 3 | 2 |
| 1.05099 | 13 | 2 | 3 | 5+ | 1.05014 | 12 | 3 | 6 | 4 | 1.04555 | 5 | 6 | 5 | 1 |
| 1.04396 | 14 | 4 | 7 | 3 | 1.03661 | 5 | 1 | 8 | 4 | 1.03563 | 8 | 0 | 10 | 3 |
| 1.02908 | 7 | 5 | 7 | 2 | 1.02441 | 5 | 3 | 1 | 5 | 1.02258 | 10 | 6 | 6 | 0 |
| 1.02110 | 12 | 3 | 10 | 2 | 1.01234 | 30 | 0 | 12 | 1+ | 1.00658 | 6 | 6 | 6 | 1 |
| 1.00396 | 20 | 3 | 7 | 4 | 1.00152 | 56 | 2 | 11 | 2+ | 0.99645 | 12 | 4 | 10 | 1+ |
| 0.99452 | 17 | 7 | 0 | 1+ | 0.99171 | 22 | 5 | 2 | 4+ | 0.98102 | 5 | 7 | 3 | 0 |
| 0.97928 | 11 | 5 | 8 | 2 | 0.97740 | 11 | 4 | 6 | 4 | 0.96569 | 22 | 6 | 7 | 1 |
| 0.96273 | 30 | 6 | 6 | 2 | 0.96002 | 10 | 7 | 4 | 0 | 0.95756 | 5 | 3 | 8 | 4 |
| 0.95655 | 8 | 4 | 1 | 5 | 0.95453 | 5 | 5 | 7 | 3 | 0.95385 | 11 | 4 | 10 | 2 |
| 0.95193 | 18 | 0 | 0 | 6 | 0.94984 | 18 | 1 | 7 | 5M | 0.94984 | 18 | 7 | 1 | 2M |
| 0.94731 | 5 | 4 | 11 | 0 | 0.94148 | 11 | 4 | 9 | 3M | 0.94148 | 11 | 7 | 2 | 2M |
| 0.93983 | 6 | 4 | 7 | 4 | 0.93454 | 13 | 4 | 11 | 1 | 0.93374 | 9 | 0 | 10 | 4 |
| 0.93070 | 17 | 5 | 9 | 2M | 0.93070 | 17 | 5 | 5 | 4M | 0.92827 | 25 | 1 | 13 | 1+ |
| 0.92528 | 20 | 2 | 7 | 5 | 0.91791 | 21 | 0 | 8 | 5M | 0.91791 | 21 | 5 | 10 | 1M |
| 0.91631 | 18 | 2 | 1 | 6M | 0.91631 | 18 | 4 | 4 | 5M | 0.91003 | 23 | 7 | 4 | 2+ |
Table 16. X-ray diffraction pattern of BaY2ZnO5 with d spacings (A), Miller indices h k l, and integrated intensities I normalized to the value of 999 as the maximum.
| d | I | h | k | l | d | I | h | k | l | d | I | h | k | l |
| 6.16838 | 7 | 0 | 2 | 0 | 6.13423 | 32 | 1 | 1 | 0 | 4.18582 | 15 | 0 | 2 | 1M |
| 4.18582 | 15 | 1 | 1 | 1M | 3.60450 | 6 | 1 | 2 | 1 | 3.55471 | 11 | 1 | 3 | 0 |
| 3.53510 | 14 | 2 | 0 | 0 | 3.39833 | 57 | 2 | 1 | 0 | 3.08419 | 112 | 0 | 4 | 0 |
| 3.06712 | 40 | 2 | 2 | 0 | 3.01758 | 999 | 1 | 3 | 1 | 2.92015 | 831 | 2 | 1 | 1 |
| 2.85454 | 429 | 0 | 0 | 2 | 2.82693 | 202 | 1 | 4 | 0 | 2.71354 | 33 | 0 | 4 | 1 |
| 2.70189 | 144 | 2 | 2 | 1 | 2.58804 | 46 | 1 | 1 | 2 | 2.53336 | 161 | 1 | 4 | 1 |
| 2.43245 | 10 | 1 | 2 | 2 | 2.42654 | 163 | 2 | 3 | 1 | 2.32957 | 115 | 1 | 5 | 0+ |
| 2.31487 | 17 | 3 | 1 | 0 | 2.22180 | 53 | 1 | 3 | 2M | 2.22180 | 53 | 2 | 0 | 2M |
| 2.20152 | 194 | 3 | 2 | 0 | 2.18575 | 101 | 2 | 1 | 2 | 2.17842 | 25 | 3 | 0 | 1 |
| 2.15692 | 33 | 1 | 5 | 1 | 2.14523 | 90 | 3 | 1 | 1 | 2.09495 | 121 | 0 | 4 | 2 |
| 2.08958 | 24 | 2 | 2 | 2 | 2.05563 | 82 | 0 | 6 | 0M | 2.05563 | 82 | 3 | 2 | 1 |
| 2.00863 | 328 | 1 | 4 | 2 | 1.95412 | 74 | 2 | 3 | 2 | 1.92500 | 9 | 3 | 3 | 1 |
| 1.90705 | 26 | 2 | 5 | 1 | 1.87261 | 135 | 3 | 4 | 0 | 1.86591 | 19 | 1 | 6 | 1 |
| 1.80483 | 96 | 1 | 5 | 2 | 1.77828 | 70 | 3 | 4 | 1M | 1.77828 | 70 | 2 | 6 | 0M |
| 1.76755 | 84 | 4 | 0 | 0 | 1.74968 | 37 | 4 | 1 | 0 | 1.74329 | 171 | 3 | 2 | 2 |
| 1.71007 | 49 | 1 | 7 | 0 | 1.69702 | 21 | 2 | 6 | 1 | 1.67773 | 166 | 1 | 3 | 3 |
| 1.67288 | 37 | 4 | 1 | 1 | 1.66838 | 23 | 0 | 6 | 2 | 1.66041 | 159 | 2 | 1 | 3 |
| 1.63816 | 64 | 1 | 7 | 1 | 1.63302 | 17 | 3 | 5 | 1 | 1.62383 | 15 | 4 | 3 | 0M |
| 1.62383 | 15 | 1 | 6 | 2M | 1.61954 | 6 | 0 | 4 | 3 | 1.61705 | 29 | 2 | 2 | 3 |
| 1.57842 | 48 | 1 | 4 | 3M | 1.57842 | 48 | 2 | 7 | 0M | 1.56576 | 141 | 3 | 4 | 2 |
| 1.55177 | 43 | 2 | 3 | 3 | 1.54935 | 25 | 3 | 6 | 0 | 1.52030 | 75 | 2 | 7 | 1 |
| 1.50829 | 59 | 2 | 6 | 2M | 1.50829 | 59 | 1 | 8 | 0M | 1.50278 | 37 | 4 | 0 | 2 |
| 1.49175 | 34 | 4 | 1 | 2 | 1.48874 | 48 | 0 | 8 | 1 | 1.48100 | 45 | 4 | 4 | 1M |
| 1.48100 | 45 | 3 | 0 | 3M | 1.47379 | 8 | 1 | 5 | 3 | 1.47004 | 22 | 3 | 1 | 3 |
| 1.46697 | 19 | 1 | 7 | 2 | 1.45680 | 30 | 1 | 8 | 1 | 1.43970 | 8 | 3 | 2 | 3 |
| 1.43689 | 14 | 4 | 5 | 0 | 1.42727 | 78 | 0 | 0 | 4 | 1.41144 | 61 | 4 | 3 | 2M |
| 1.41144 | 61 | 3 | 7 | 0M | 1.39344 | 32 | 4 | 5 | 1 | 1.38053 | 24 | 2 | 7 | 2 |
| 1.37829 | 19 | 5 | 2 | 0 | 1.37257 | 12 | 5 | 0 | 1 | 1.37016 | 6 | 1 | 6 | 3+ |
| 1.36415 | 8 | 5 | 1 | 1 | 1.36170 | 40 | 3 | 6 | 2 | 1.34036 | 13 | 4 | 6 | 0 |
| 1.33476 | 11 | 3 | 4 | 3 | 1.33246 | 9 | 1 | 8 | 2 | 1.31592 | 6 | 2 | 1 | 4 |
| 1.30980 | 14 | 1 | 9 | 1 | 1.30196 | 84 | 5 | 3 | 1 | 1.29894 | 7 | 2 | 6 | 3 |
| 1.29530 | 12 | 0 | 4 | 4 | 1.29040 | 8 | 3 | 8 | 0 | 1.28802 | 16 | 4 | 1 | 3 |
| 1.28346 | 12 | 4 | 5 | 2 | 1.27804 | 7 | 2 | 9 | 0 | 1.27409 | 28 | 1 | 4 | 4 |
| 1.27197 | 25 | 1 | 7 | 3 | 1.26956 | 5 | 3 | 5 | 3 | 1.26519 | 17 | 3 | 7 | 2 |
| 1.26047 | 6 | 5 | 1 | 2 | 1.25865 | 27 | 3 | 8 | 1 | 1.24760 | 16 | 4 | 7 | 0M |
| 1.24760 | 16 | 2 | 9 | 1M | 1.24118 | 18 | 5 | 2 | 2 | 1.23368 | 5 | 0 | 10 | 0 |
| 1.22685 | 7 | 5 | 5 | 0 | 1.21923 | 26 | 4 | 7 | 1 | 1.21707 | 33 | 1 | 9 | 2M |
| 1.21707 | 33 | 1 | 5 | 4M | 1.21412 | 44 | 2 | 7 | 3M | 1.21412 | 44 | 4 | 6 | 2M |
| 1.20584 | 11 | 0 | 10 | 1 | 1.19778 | 63 | 0 | 8 | 3M | 1.19778 | 63 | 3 | 2 | 4M |
| 1.19409 | 18 | 4 | 4 | 3 | 1.18127 | 15 | 1 | 8 | 3 | 1.17837 | 7 | 6 | 0 | 0 |
| 1.17584 | 8 | 3 | 8 | 2 | 1.17248 | 17 | 0 | 6 | 4 | 1.16646 | 5 | 2 | 9 | 2 |
| 1.16479 | 8 | 2 | 10 | 0 | 1.16201 | 6 | 4 | 8 | 0 | 1.14673 | 17 | 4 | 5 | 3 |
| 1.14351 | 8 | 4 | 7 | 2 | 1.14158 | 7 | 5 | 6 | 1 | 1.13867 | 5 | 4 | 8 | 1 |
| 1.13512 | 49 | 3 | 4 | 4M | 1.13512 | 49 | 5 | 0 | 3M | 1.13436 | 10 | 6 | 2 | 1 |
| 1.13255 | 21 | 6 | 3 | 0M | 1.13255 | 21 | 0 | 10 | 2M | 1.12715 | 10 | 5 | 5 | 2 |
| 1.11819 | 13 | 1 | 10 | 2 | 1.11286 | 12 | 2 | 6 | 4 | 1.11044 | 29 | 4 | 0 | 4 |
| 1.10597 | 12 | 4 | 1 | 4 | 1.09874 | 8 | 1 | 9 | 3 | 1.09576 | 20 | 1 | 7 | 4 |
| 1.09410 | 52 | 5 | 3 | 3 | 1.08921 | 18 | 6 | 0 | 2 | 1.08711 | 36 | 1 | 3 | 5 |
| 1.08499 | 7 | 6 | 1 | 2 | 1.08244 | 48 | 5 | 7 | 1M | 1.08244 | 48 | 2 | 1 | 5M |
| 1.07626 | 7 | 4 | 8 | 2 | 1.07306 | 14 | 3 | 10 | 1+ | 1.07306 | 14 | 6 | 2 | 2+ |
| 1.07007 | 6 | 2 | 2 | 5 | 1.06902 | 28 | 2 | 11 | 0 | 1.06802 | 16 | 3 | 8 | 3 |
| 1.06421 | 9 | 4 | 9 | 1 | 1.05857 | 14 | 1 | 4 | 5M | 1.05857 | 14 | 2 | 7 | 4M |
| 1.05290 | 19 | 6 | 3 | 2 | 1.05007 | 25 | 2 | 3 | 5M | 1.05007 | 25 | 3 | 6 | 4M |
| 1.04362 | 20 | 4 | 7 | 3 | 1.03617 | 8 | 1 | 8 | 4 | 1.03519 | 8 | 0 | 10 | 3 |
| 1.02704 | 5 | 6 | 4 | 2 | 1.02402 | 6 | 3 | 1 | 5 | 1.02237 | 13 | 6 | 6 | 0 |
| 1.02072 | 14 | 3 | 10 | 2 | 1.01198 | 31 | 4 | 5 | 4+ | 1.01198 | 31 | 0 | 12 | 1+ |
| 1.00650 | 11 | 7 | 1 | 0M | 1.00650 | 11 | 6 | 6 | 1M | 1.00357 | 23 | 3 | 7 | 4 |
| 1.00112 | 61 | 2 | 11 | 2 | 0.99612 | 11 | 4 | 10 | 1 | 0.99458 | 13 | 7 | 0 | 1 |
| 0.99143 | 21 | 5 | 2 | 4M | 0.99143 | 21 | 7 | 1 | 1M | 0.98889 | 6 | 6 | 2 | 3 |
| 0.97900 | 13 | 5 | 8 | 2 | 0.97706 | 12 | 4 | 6 | 4 | 0.97488 | 5 | 3 | 4 | 5 |
| 0.96547 | 25 | 6 | 7 | 1 | 0.96250 | 37 | 6 | 6 | 2 | 0.95987 | 9 | 7 | 4 | 0 |
| 0.95719 | 7 | 3 | 8 | 4 | 0.95622 | 7 | 4 | 1 | 5 | 0.95378 | 20 | 5 | 7 | 3M |
| 0.95378 | 20 | 4 | 10 | 2M | 0.95165 | 25 | 2 | 9 | 4M | 0.95165 | 25 | 0 | 0 | 6M |
| 0.94950 | 18 | 1 | 7 | 5M | 0.94950 | 18 | 7 | 1 | 2M | 0.94121 | 12 | 4 | 9 | 3M |
| 0.94121 | 12 | 7 | 2 | 2M | 0.93950 | 7 | 4 | 7 | 4 | 0.93422 | 9 | 4 | 11 | 1 |
| 0.93334 | 7 | 0 | 10 | 4 | 0.93042 | 18 | 5 | 5 | 4M | 0.93042 | 18 | 3 | 12 | 1M |
Table 17. X-ray diffraction pattern of BaEr2ZnO5 with d spacings (A), Miller indices h k l, and integrated intensities I normalized to the value of 999 as the maximum.
| d | I | h | k | l | d | I | h | k | l | d | I | h | k | l |
| 6.14905 | 17 | 0 | 2 | 0 | 6.11312 | 42 | 1 | 1 | 0 | 4.63267 | 8 | 1 | 2 | 0 |
| 4.42851 | 70 | 1 | 0 | 1 | 4.17113 | 5 | 0 | 2 | 1M | 4.17113 | 5 | 1 | 1 | 1M |
| 3.59356 | 13 | 1 | 2 | 1 | 3.52257 | 17 | 2 | 0 | 0 | 3.38640 | 73 | 2 | 1 | 0 |
| 3.07453 | 68 | 0 | 4 | 0 | 3.05656 | 21 | 2 | 2 | 0 | 3.00833 | 999 | 1 | 3 | 1 |
| 2.91057 | 809 | 2 | 1 | 1 | 2.84705 | 596 | 0 | 0 | 2 | 2.81788 | 216 | 1 | 4 | 0 |
| 2.70535 | 134 | 0 | 4 | 1 | 2.69308 | 63 | 2 | 2 | 1 | 2.67169 | 24 | 2 | 3 | 0 |
| 2.52554 | 106 | 1 | 4 | 1 | 2.42561 | 9 | 1 | 2 | 2 | 2.41869 | 152 | 2 | 3 | 1 |
| 2.32217 | 81 | 1 | 5 | 0 | 2.31633 | 5 | 2 | 4 | 0 | 2.30670 | 18 | 3 | 1 | 0 |
| 2.21425 | 31 | 2 | 0 | 2 | 2.19384 | 150 | 3 | 2 | 0 | 2.17921 | 94 | 2 | 1 | 2 |
| 2.17099 | 68 | 3 | 0 | 1 | 2.15023 | 33 | 1 | 5 | 1 | 2.14560 | 8 | 2 | 4 | 1 |
| 2.13794 | 81 | 3 | 1 | 1 | 2.08896 | 69 | 0 | 4 | 2 | 2.08330 | 20 | 2 | 2 | 2 |
| 2.04922 | 72 | 0 | 6 | 0M | 2.04922 | 72 | 3 | 2 | 1M | 2.00278 | 305 | 1 | 4 | 2 |
| 1.96808 | 9 | 1 | 6 | 0 | 1.94822 | 140 | 2 | 3 | 2 | 1.92854 | 7 | 0 | 6 | 1 |
| 1.91855 | 23 | 3 | 3 | 1 | 1.90096 | 12 | 2 | 5 | 1 | 1.86625 | 141 | 3 | 4 | 0 |
| 1.86011 | 24 | 1 | 6 | 1 | 1.83269 | 5 | 1 | 0 | 3 | 1.79950 | 55 | 1 | 5 | 2 |
| 1.77241 | 43 | 3 | 4 | 1M | 1.77241 | 43 | 2 | 6 | 0M | 1.76129 | 70 | 4 | 0 | 0 |
| 1.74350 | 27 | 4 | 1 | 0 | 1.73777 | 120 | 3 | 2 | 2 | 1.70467 | 63 | 1 | 7 | 0 |
| 1.69162 | 14 | 2 | 6 | 1 | 1.67310 | 168 | 1 | 3 | 3 | 1.66710 | 36 | 4 | 1 | 1 |
| 1.66344 | 25 | 0 | 6 | 2 | 1.65570 | 144 | 2 | 1 | 3 | 1.63306 | 51 | 1 | 7 | 1 |
| 1.62765 | 11 | 3 | 5 | 1 | 1.61893 | 12 | 1 | 6 | 2 | 1.61506 | 23 | 0 | 4 | 3 |
| 1.61244 | 12 | 2 | 2 | 3 | 1.57423 | 22 | 1 | 4 | 3 | 1.56081 | 153 | 3 | 4 | 2 |
| 1.54731 | 40 | 2 | 3 | 3 | 1.54422 | 21 | 3 | 6 | 0 | 1.53726 | 8 | 0 | 8 | 0 |
| 1.51548 | 108 | 2 | 7 | 1 | 1.50416 | 38 | 2 | 6 | 2 | 1.50192 | 7 | 1 | 8 | 0 |
| 1.49784 | 55 | 4 | 0 | 2 | 1.48685 | 16 | 4 | 1 | 2 | 1.48413 | 37 | 0 | 8 | 1 |
| 1.47607 | 82 | 3 | 0 | 3M | 1.47607 | 82 | 4 | 4 | 1M | 1.46944 | 10 | 1 | 5 | 3M |
| 1.46944 | 10 | 2 | 4 | 3M | 1.46565 | 20 | 3 | 1 | 3 | 1.46255 | 39 | 1 | 7 | 2 |
| 1.45225 | 30 | 1 | 8 | 1 | 1.43539 | 5 | 3 | 2 | 3 | 1.43200 | 13 | 4 | 5 | 0 |
| 1.42352 | 93 | 0 | 0 | 4 | 1.40679 | 45 | 4 | 3 | 2M | 1.40679 | 45 | 3 | 7 | 0M |
| 1.39987 | 5 | 5 | 1 | 0 | 1.38878 | 31 | 3 | 3 | 3M | 1.38878 | 31 | 4 | 5 | 1M |
| 1.37628 | 6 | 2 | 7 | 2 | 1.37343 | 13 | 5 | 2 | 0 | 1.36777 | 11 | 5 | 0 | 1 |
| 1.36615 | 8 | 1 | 6 | 3M | 1.36615 | 8 | 3 | 7 | 1M | 1.35741 | 32 | 3 | 6 | 2 |
| 1.35267 | 7 | 0 | 8 | 2 | 1.33585 | 15 | 4 | 6 | 0+ | 1.33132 | 9 | 5 | 3 | 0M |
| 1.33132 | 9 | 3 | 4 | 3M | 1.32841 | 5 | 1 | 8 | 2 | 1.31229 | 7 | 2 | 1 | 4 |
| 1.30571 | 7 | 1 | 9 | 1 | 1.29746 | 92 | 5 | 3 | 1 | 1.29178 | 6 | 0 | 4 | 4 |
| 1.28400 | 13 | 4 | 1 | 3 | 1.27929 | 11 | 4 | 5 | 2 | 1.27060 | 37 | 1 | 4 | 4 |
| 1.26825 | 19 | 1 | 7 | 3 | 1.26121 | 18 | 3 | 7 | 2 | 1.25625 | 16 | 2 | 3 | 4M |
| 1.25625 | 16 | 5 | 1 | 2M | 1.25459 | 32 | 3 | 8 | 1 | 1.24323 | 6 | 2 | 9 | 1 |
| 1.23702 | 15 | 5 | 2 | 2 | 1.22981 | 6 | 0 | 10 | 0 | 1.22262 | 6 | 5 | 5 | 0 |
| 1.21520 | 24 | 4 | 7 | 1 | 1.21364 | 15 | 1 | 5 | 4 | 1.21076 | 44 | 2 | 7 | 3 |
| 1.20934 | 11 | 4 | 6 | 2 | 1.20209 | 17 | 0 | 10 | 1 | 1.19430 | 45 | 0 | 8 | 3M |
| 1.19430 | 45 | 3 | 2 | 4M | 1.19036 | 29 | 4 | 4 | 3 | 1.17778 | 15 | 1 | 8 | 3 |
| 1.16915 | 19 | 0 | 6 | 4M | 1.16915 | 19 | 6 | 1 | 0M | 1.16108 | 5 | 2 | 10 | 0 |
| 1.15817 | 6 | 4 | 8 | 0 | 1.14315 | 20 | 4 | 5 | 3 | 1.13982 | 6 | 4 | 7 | 2 |
| 1.13771 | 6 | 5 | 6 | 1M | 1.13771 | 6 | 2 | 10 | 1M | 1.13493 | 5 | 4 | 8 | 1 |
| 1.13184 | 46 | 3 | 4 | 4+ | 1.12890 | 21 | 0 | 10 | 2M | 1.12890 | 21 | 6 | 3 | 0M |
| 1.12342 | 6 | 5 | 5 | 2+ | 1.11476 | 15 | 1 | 10 | 2 | 1.10968 | 9 | 2 | 6 | 4 |
| 1.10713 | 29 | 4 | 0 | 4 | 1.10267 | 7 | 4 | 1 | 4 | 1.09919 | 7 | 5 | 7 | 0 |
| 1.09547 | 5 | 1 | 9 | 3 | 1.09265 | 25 | 1 | 7 | 4+ | 1.09059 | 54 | 5 | 3 | 3 |
| 1.08949 | 8 | 4 | 2 | 4M | 1.08949 | 8 | 3 | 10 | 0M | 1.08550 | 17 | 6 | 0 | 2 |
| 1.08416 | 48 | 1 | 3 | 5M | 1.08416 | 48 | 1 | 11 | 1M | 1.08129 | 9 | 6 | 1 | 2 |
| 1.07939 | 44 | 2 | 1 | 5M | 1.07939 | 44 | 5 | 7 | 1M | 1.07512 | 8 | 2 | 10 | 2 |
| 1.07280 | 11 | 4 | 8 | 2 | 1.06897 | 5 | 6 | 2 | 2 | 1.06766 | 9 | 0 | 4 | 5M |
| 1.06766 | 9 | 2 | 2 | 5M | 1.06563 | 32 | 2 | 11 | 0 | 1.06475 | 20 | 3 | 8 | 3 |
| 1.05585 | 6 | 1 | 4 | 5 | 1.04933 | 21 | 6 | 3 | 2 | 1.04762 | 9 | 2 | 3 | 5 |
| 1.04683 | 14 | 2 | 11 | 1M | 1.04683 | 14 | 3 | 6 | 4M | 1.04036 | 15 | 4 | 7 | 3 |
| 1.03210 | 10 | 0 | 10 | 3 | 1.02542 | 14 | 5 | 7 | 2 | 1.02115 | 7 | 3 | 1 | 5 |
| 1.01885 | 12 | 6 | 6 | 0 | 1.01751 | 15 | 3 | 10 | 2 | 1.00957 | 7 | 4 | 5 | 4 |
| 1.00856 | 22 | 0 | 12 | 1M | 1.00856 | 22 | 4 | 10 | 0M | 1.00299 | 8 | 7 | 1 | 0M |
| 1.00299 | 8 | 6 | 6 | 1M | 1.00061 | 23 | 3 | 7 | 4 | 0.99801 | 66 | 2 | 11 | 2+ |
| 0.99288 | 13 | 4 | 10 | 1+ | 0.99109 | 15 | 7 | 0 | 1 | 0.98817 | 18 | 5 | 2 | 4M |
| 0.98817 | 18 | 7 | 1 | 1M | 0.97580 | 11 | 5 | 8 | 2 | 0.97411 | 12 | 4 | 6 | 4 |
| 0.96219 | 26 | 6 | 7 | 1 | 0.95928 | 27 | 6 | 6 | 2 | 0.95650 | 9 | 7 | 4 | 0 |
| 0.95439 | 9 | 1 | 11 | 3M | 0.95439 | 9 | 3 | 8 | 4M | 0.95345 | 7 | 4 | 1 | 5 |
| 0.95048 | 12 | 4 | 10 | 2+ | 0.94902 | 19 | 0 | 0 | 6 | 0.94695 | 9 | 1 | 7 | 5 |
| 0.93095 | 16 | 4 | 11 | 1M | 0.93095 | 16 | 0 | 10 | 4M | 0.92746 | 12 | 5 | 5 | 4M |
| 0.92746 | 12 | 5 | 9 | 2M | 0.92516 | 16 | 6 | 5 | 3M | 0.92516 | 16 | 1 | 13 | 1M |
Acknowledgments
The authors acknowledge the International Center for Diffraction Data for partial financial support through the Grant-in-Aid program.
References
- G. Xiao, F. H. Streita, A. Gavrin, Y. W. Du, and C. L. Chien, Effect of Transition-Metal Elements on the Superconductivity of YBaCuO, Phys. Rev. B 35, 8782 – 8784 (1992).
- W. Wong-Ng, D. Kaiser, F. Gale, S. F. Watkins, and F. Fronczek, X-Ray Crystallographic Studies of a Thermomechanically Detwinned Single Crystal of Ba2YCu3O6+ x , Phys Rev B 41, 4220 – 4226 (1990).
- M. Z. Cieplak, G. Xiao, C. L. Chien, A. Bakhshai, D. Artymowicz, W. Bryden, J. K. Stalick, and J. J. Rhyne, Incorporation of Gold into YBa2Cu3O7 : Structure and Tc Enhancement, Phys. Rev. B 42, 6200 – 6208 (1990).
- P. F. Miceli, J. M. Tarascon, L. H. Greene, P. Barboux, F. J. Rotella, and J. D. Jorgensen, Role of Bond Lengths in the 90-K Superconductor: A Neutron Powder-Diffraction Study of YBa2Cu3− x Co x O7− y, Phys. Rev. B 37 (10), 5932 – 5935 (1988).
- C. Michel, L. Er-Rakho, and B. Raveau, Les Oxydes La4−2 x Ba2+2 x Cu2− x O10−2 x : Une Structure Inedite Constituee de Groupements CuO4 Carres Plans Isoles, J. Solid State Chem. 39, 161 – 167 (1981).
- C. Michel, L. Er-Rakho, and B. Raveau, Une Nouvelle Famille Structurale: les Oxydes Ln4−2 x Ba2+2 x Zn2− x O10−2 x (Ln=La, Nd), J. Solid State Chem. 42, 176 – 182 (1982).
- C .Michel and B. Raveau, Les Oxydes A2 BaCuO5 (A=Y, Sm, Eu, Gd, Dy, Ho, Er, Yb), J. Solid State Chem. 43, 73 – 80 (1982).
- [8] C. Michel and B. Raveau, Ln2 BaZnO5 and Ln2 BaZn1− x Cu x O5: A Series of Zinc Oxides with Zinc in a Pyramidal Coordination, J. Solid State Chem. 49, 150 – 156 (1983).
- W. Wong-Ng, B. H. Toby, and W. Greenwood, Crystallization Studies of BaR2ZnO5 (R=La, Nd, Ho, Er, and Y), Powd. Diffr. 13 (3), 144 – 151 (1998).
- Powder Diffraction File (PDF), produced by International Centre for Diffraction Data (ICDD), Newtown Square, 12 Campus Blvd., Newtown Square, PA 19073-3273.
- H. M. Rietveld, A Profile Refinement Method for Nuclear and Magnetic Structures, J. Appl. Cryst. 2, 65 – 71 (1969).
- A. C. Larson and R. B. von Dreele, GSAS The General Structure Analysis System, U.S. Government contract (W-7405-ENG-36) by the Los Alamos National Laboratory, which is operated by the University of California for the U.S. Department of Energy, VMS version.
- Inorganic Structure Data Base (ICSD), published by Fachinformationszentrum Karlsruhe (1994); Collection Code 406331.
- C. R. Sfreddo and H. Mueller-Buschbaum, Zur Kristallchemie von Oxozinkat-Platinaten und Oxozinkaten der Zusammensetzung Ba17Tm16 Zn8 Pt4O37 and Ba5Zn4 Tm8O2 1, Zeit. Anorg. Allg. Chem. 623, 699 – 704 (1997).
- R. D. Shannon and C. T. Prewitt, Effective Ionic Radii in Oxides and Fluorides, Acta Cryst. 25, 925 – 946 (1969).
- R. D. Shannon, Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides, Acta Cryst. A 32, 751 – 767 (1976).
- C. Michel and B. Raveau, Copper Doped Zinc Oxide Y2BaZnO5 : ESR and Optical Investigation, Mat. Res. Bull. 19, 849 – 855 (1984).
- M. Taibi, J. Aride, J. Darriet, A. Moqine, and A. Boukhari, Structure Cristalline de l'oxyde Nd2 BaZnO5, J. Solid State Chem. 86, 233 – 237 (1990).
- J. K. Stalick and W. Wong-Ng, Neutron Diffraction Study of the `Brown Phase' BaNd2CuO5, Materials Letters 9 (10), 401 – 405 (1990).
- I. D. Brown and D. Altermatt, Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database, Acta Cryst. B 41, 244 – 247 (1995).
- N. E. Brese and M. O'Keeffe, Bond-Valence Parameters for Solids, Acta Crystallogr. B 47, 192 – 199 (1991).
- R. M. Hazen, L. W. Finger, R. A. Angel, C. T. Prewitt, N. L. Ross, H. K. Mao, and C. G. Hadidiacos, Crystallographic Description of Phases in the Y-Ba-Cu-O Superconductor, Phys. Rev. B 35, 7238 – 7241 (1987).
- S. F. Watkins, F. R. Fronczek, K. S. Wheelock, R. G. Goodrich, W. O. Hamilton, and W. W. Johnson, The Crystal Structure of Y2BaCuO5, Acta Cryst. C 44, 3 – 6 (1988).
- W. Wong-Ng, M. Kuchinskin, B. Paretzkin, and H. F. McMurdie, Crystal Chemistry and Phase Equilibrium Studies of BaO-1/2R2O3 -CuO. I. X-Ray Powder Characterization of BaR2CuO5 and Related Compounds, Powd. Diff. 4 (1), 2 – 8 (1989).
About the authors: James Kaduk is an assoicate research scientist with BP Amoco PLC corporation. He works extensively in the research areas of x-ray and neutron diffraction, including powder and single crystal crystallography. Winnie Wong-Ng is a research chemist in the Phase Equilibria group of the Ceramics Division of the NIST Materials Science and Engineering Laboratory. She has been actively engaged in research on the phase equilibria, crystallography and crystal chemistry of high Tc superconductor materials since 1986. Brian Toby is the leader of the crystallography team of the Radiation Reactor Division of the NIST Materials Science and Engineering Laboratory. William Greenwood was a graduate student, and Jeremy Dillingham is a senior student from the Geology Department of the University of Maryland. The National Institute of Standards and Technology is an agency of the Technology Administration, U.S. Department of Commerce.